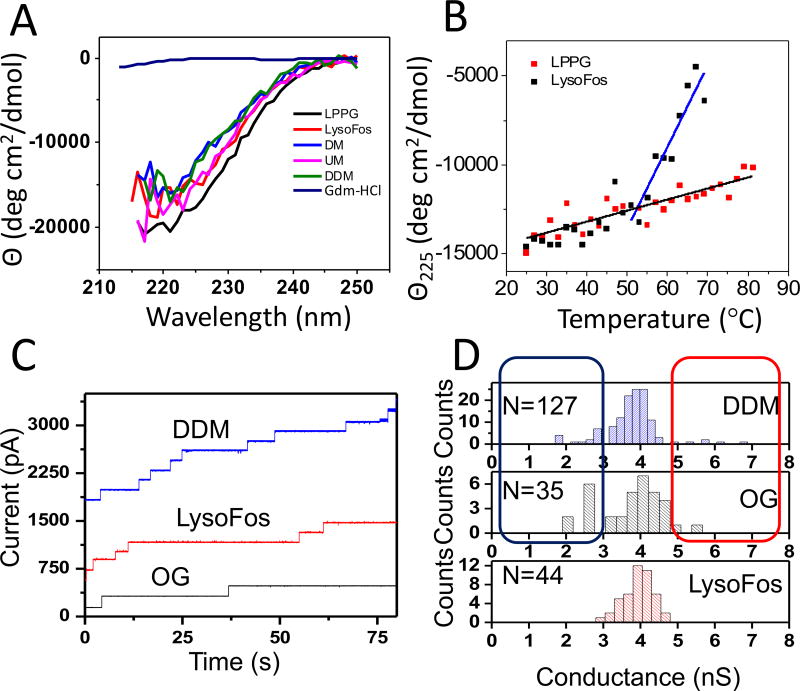
Figure 4. Unusual thermostability of LPPG-refolded protein nanopores and functional reconstitution of LysoFos-, DDM-, and OG-refolded protein nanopores.
(A) Wavelength circular dichroism scans of ~1 µM FhuA ΔC/Δ5L in 200 mM NaCl, 50 mM potassium phosphate, pH 7.4 with 20 mM of the specified detergent. In the negative control experiment, we used a buffer solution containing 6 M Gdm-HCl; (B) Temperature-dependent ellipticity θ225 of FhuA ΔC/Δ5L in either 20 mM LPPG or in 20 mM LysoFos. For DDM, UM, and DM, we could not achieve a sufficiently high aggregation-free protein concentration; (C) Representative step-wise insertions of single nanopores, over at least 6 distinct experiments, after the addition of DDM- (blue), OG- (black), or LysoFos-refolded (red) FhuA ΔC/Δ5L at an applied transmembrane potential of +40 mV. 40 µl pure and denatured 6×His+-tagged FhuA ΔC/Δ5L was 50-fold diluted into 29 mM DDM, 85 mM OG, or 16 mM LysoFos, containing 200 mM NaCl, 50 mM Tris.HCl, 1 mM EDTA, pH 8.0. The dilution ratio of the refolded protein within the bilayer chamber was ~ 1:1000. Therefore, the presence of detergent within the bilayer chamber did not affect the stability of the membrane;45 (D) The unitary-conductance histograms of DDM-, OG-, and LysoFos-refolded FhuA ΔC/Δ5L. The electrical recordings were collected using 1M KCl, 10 mM potassium phosphate, pH 7.4.