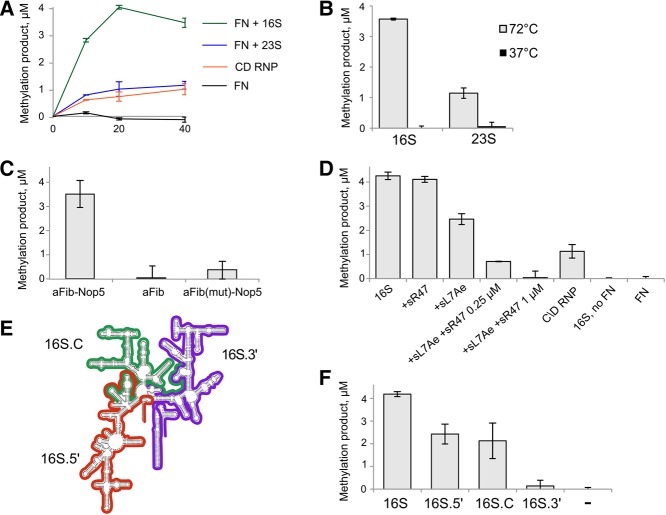
FIGURE 1.
P. abyssi aFib–Nop5 heterodimer methylates P. abyssi 16S and 23S rRNA. Reactions containing 1 µM aFib–Nop5 (FN), 100 µM [methyl-3H]-SAM, and 1 µM in vitro transcribed substrate RNAs (as indicated) were incubated at 72 °C for 40 min. C/D RNP reactions contained 1 µM C/D RNP, assembled with sR47 guide RNA, and substrate tRNA-Leu(CAA). (A) [methyl-3H]-incorporation time courses of the full C/D RNP and aFib–Nop5 with 16S or 23S rRNAs. (B) Temperature dependence of the aFib–Nop5 methylation activity. (C) Methylation activity of a separate aFib protein and aFib–Nop5 and mutant aFib(D150A)–Nop5 heterodimers. (D) aFib–Nop5 activity on 16S rRNA is inhibited by the assembly of a full C/D RNP. aFib–Nop5 and 16S rRNA reaction was supplemented with 3 µM L7Ae and either 0.25 µM or 0.5 µM sR47 C/D guide RNA. Reactions with only one additional C/D RNP component contained either 3 µM L7Ae or 1 µM sR47. (E) Three truncated P. abyssi 16S rRNA substrates: 16S.5′—red, 16S.C—green, and 16S.3′—purple. Secondary structure map according to Cannone et al. (2002). (F) aFib–Nop5 activity on truncated 16S rRNA substrates 16S.5′, 16S.C, and 16S.3′.