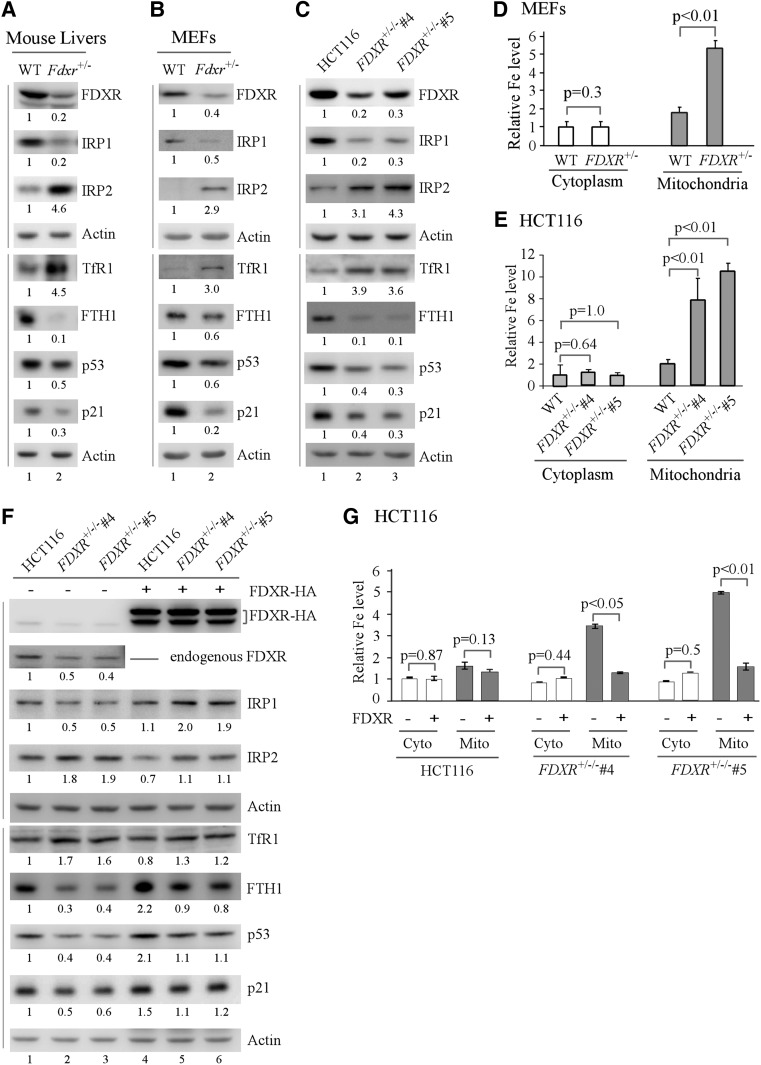
Figure 3.
Iron metabolism and the p53 pathway are regulated by FDXR. (A–C) Western blots were prepared using extracts from wild-type and Fdxr+/− mouse livers (A) and MEFs (B) and wild-type and FDXR+/−/− HCT116 cells (C). The blots were probed with antibodies against FDXR, IRP1, IRP2, TfR1, FTH1, p53, p21, and actin, respectively. (D,E) FDXR deficiency leads to mitochondrial iron accumulation. The level of cytosolic and mitochondrial iron (Fe2+) was measured by QuantiChrom iron assay in a pair of wild-type and Fdxr+/− littermate MEFs (D) and in isogenic control and FDXR+/−/− HCT116 cells (E). The level of cytosolic iron in wild-type MEFs and isogenic control HCT116 cells (the first left column) was set at 1.0. Data are mean ± SD from three independent experiments. (F) Ectopic expression of FDXR restores near-normal expression of iron regulatory proteins and p53. Isogenic control and FDXR+/−/− HCT116 cells were transfected with 1 µg of control pcDNA3 (−) or a vector expressing FDXR with an HA tag at its C terminus (FDXR-HA) (+) for 24 h followed by Western blot analysis with various antibodies as indicated. (G) Ectopic expression of FDXR abrogates mitochondrial iron overload in FDXR-deficient cells. The experiment was performed as in F. The level of cytosolic and mitochondrial iron (Fe2+) was measured in isogenic control and FDXR+/−/− HCT116 cells transfected with control pcDNA3 (−) or a vector expressing FDXR-HA (+). (First left column) The level of cytosolic iron in isogenic control HCT116 cells transfected with control pcDNA3 was set at 1.0. Data are mean ± SD from three independent experiments.