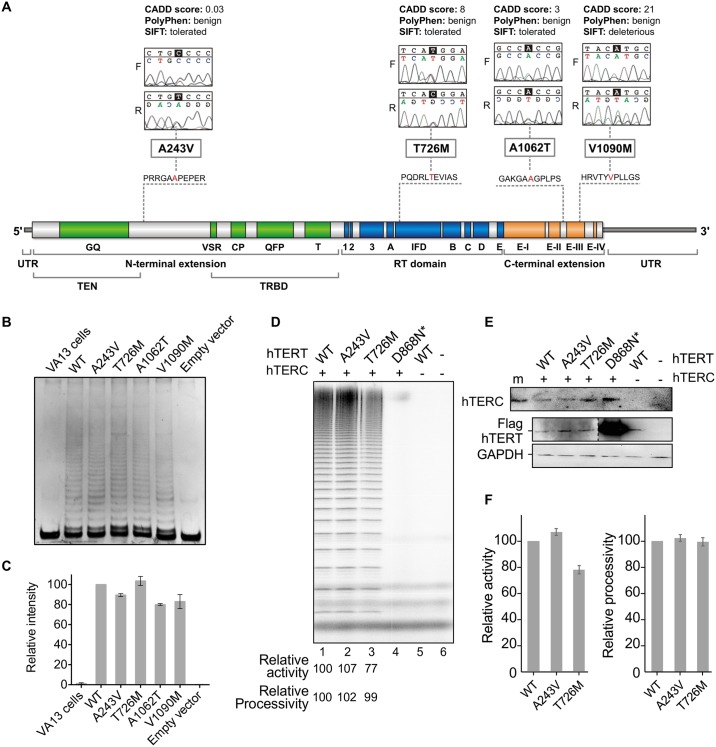
Fig 2. The functional consequences of variants on enzyme activity.
(A) Four non-synonymous TERT heterozygous mutations (A243V, T726M, A1062T, and V1090M) were detected in HCC patients in both forward and reverse sequences; in silico analysis was performed by CADD, PolyPhen, and SIFT to predict the impact of each variant on the structure and function of the enzyme. The telomerase enzyme is represented in its three domains: N-terminal (green), Reverse Transcriptase (blue), and C-terminal (orange) with all described mutations in red (adapted from http://telomerase.asu.edu/diseases.html#tert). (B) Analysis of TERT variants’ impact on telomerase activity using PCR-based TRAP assay: representative gel image of telomeric DNA repeats generated from wild-type (WT) and variant telomerases reconstituted in vivo. The cell lysates for TRAP assay were obtained from reconstitution of the WT, empty, or mutated TERT expression vectors in the telomerase-negative VA13 cell line cotransfected with TERC-containing vector. No telomeric DNA repeats were obtained from lysates of VA13 cells and cells transfected with the empty vector (negative controls). (C) Mean intensity (and standard error) of telomeric DNA repeats quantitated from the TRAP gels. Intensities are shown relative to the WT (set as 100%). Cell lysates were obtained from two independent transfections. The TRAP assay was performed for each transfection. (D) Analysis of TERT variants’ impact on telomerase activity and processivity using direct assay: gel image of telomeric DNA repeats generated from WT and variant telomerases reconstituted in vivo and immuno-purified. The decreased total intensity of the DNA repeat products generated by variant telomerases relative to wild-type enzyme reflects slightly impaired enzymatic activity of TERT T726M. Processivity remained similar to WT for the two variants tested (A243V and T726M). The TERT mutation D868N is a negative control, catalytically defective in one of the three essential aspartic acid residues for reverse transcription. (E) Northern blot for TERC levels from immuno-purified telomerases and Western blot for TERT expression levels in cells. Western blot performed with anti-Flag and anti-GAPDH antibodies for ectopically expressed Flag-tagged TERT and endogenous GAPDH, respectively. The greater intensity of the catalytically inactive D868N mutant was due to the presence of a 3×Flag tag in place of a single Flag present for the WT and variant TERT proteins. (F) Mean telomerase activity and processivity derived from four independent activity assays. Enzymes were purified from cell lysates from two separate transfections.