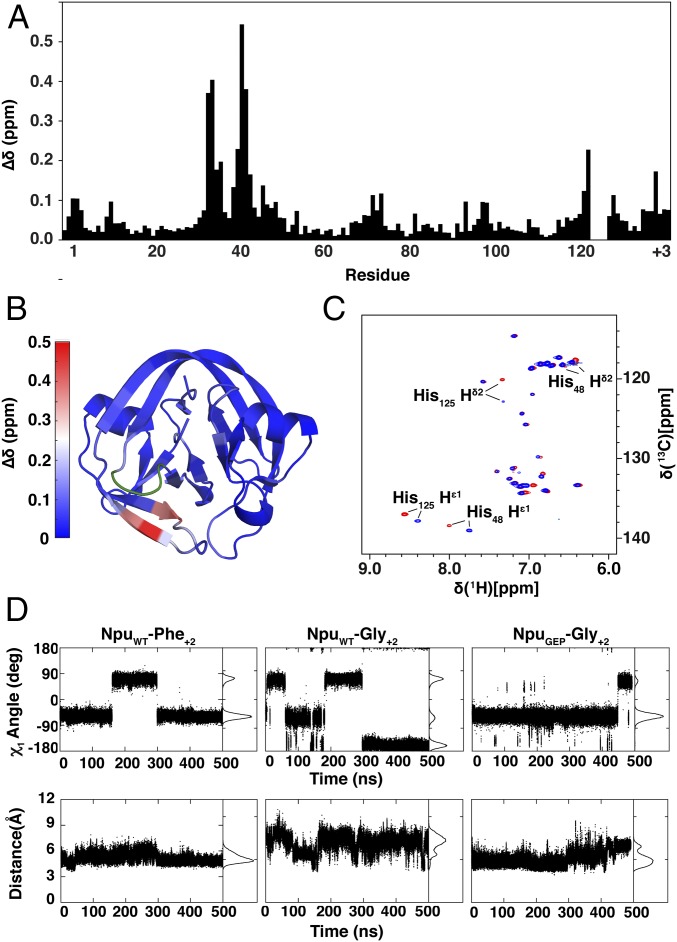
Fig. 3.
Structural and dynamic effects of the loop mutation. (A) Differences in backbone chemical shifts between NpuWT and NpuGEP in the context of a Gly+2 extein. The weighted average chemical shift perturbation (∆δ) was calculated for each residue. His125, whose backbone could not be assigned, and residues 122–124 of the His125 loop, which were mutated, were not calculated. (B) The ∆δ values from A are shown mapped onto the crystal structure of the fused Npu intein (PDB ID: 4KL5). Residues 32, 33, 40, and 41 are depicted in shades of red corresponding to the heat map key, whereas residues 122–125 are depicted in green. (C) 1H [13C]-HSQC spectra of fused NpuWT (red) and NpuGEP (blue) inteins in the context of a Gly+2 extein. (D, Top) His125 χ1 angles calculated from individual MD simulation trajectories for NpuWT-Phe+2, NpuWT-Gly+2, and NpuGEP-Gly+2 split intein complexes. (Bottom) Distance between His125Cβ and Asn137Cβ atoms calculated from respective MD simulation trajectories.