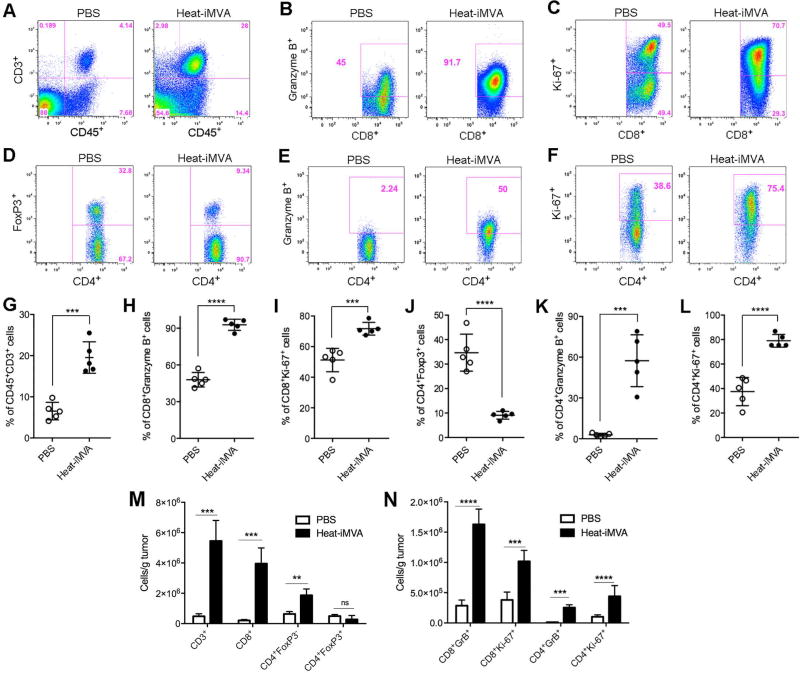
Fig. 4. Intratumoral injection of Heat-iMVA leads to immunological changes in the tumor microenvironment.
5 × 105 B16-F10 melanoma cells were implanted intradermally to the right flank of the mice. Seven days post implantation, we injected either Heat-iMVA (an equivalent of 2× 107 pfu) or PBS into the tumors on the right flank. The injection was repeated three days later. Tumors were harvested 3 days post last injection and cell suspensions were generated. The live immune cell infiltrates in the tumors were analyzed by FACS. (A-F) Representative flow cytometry plot of CD3+ CD45+ T cells (A), CD8+ cells expressing Granzyme B+ (B) or Ki-67 (C), CD4+ cells expressing FoxP3 (D), Granzyme B (E), or Ki-67 (F). (G-L) Percentages of CD45+ CD3+ (G), CD8+ Granzyme B+ (H), CD8+ Ki-67+ (I), CD4+ Foxp3+ (J), CD4+ Granzyme B+ (K), and CD4+ Ki67+ (L) cells within tumors of mice treated with PBS (n=5) or Heat-iMVA (n=5; ***P < 0.001; ****P < 0.0001, t test). A representative experiment is shown, repeated at least twice. (M) The absolute numbers of tumor infiltrating CD3+, CD8+, CD4+ FoxP3−, and CD4+ FoxP3+ cells per gram of injected tumors treated with either Heat-iMVA or PBS (n=5; **P < 0.01; ***P < 0.001, t test). (N) The absolute numbers of CD8+ Granzyme B+, CD8+ Ki67+, CD4+ Granzyme B+, and CD4+ Ki67+ cells in the injected tumors treated with either Heat-iMVA or PBS (n=5; ***P < 0.001; ****P < 0.0001, t test).