Larvaceans can filter microplastics from the water and package them into their fecal pellets, transporting them into the deep sea.
Abstract
Plastic waste is a pervasive feature of marine environments, yet little is empirically known about the biological and physical processes that transport plastics through marine ecosystems. To address this need, we conducted in situ feeding studies of microplastic particles (10 to 600 μm in diameter) with the giant larvacean Bathochordaeus stygius. Larvaceans are abundant components of global zooplankton assemblages, regularly build mucus “houses” to filter particulate matter from the surrounding water, and later abandon these structures when clogged. By conducting in situ feeding experiments with remotely operated vehicles, we show that giant larvaceans are able to filter a range of microplastic particles from the water column, ingest, and then package microplastics into their fecal pellets. Microplastics also readily affix to their houses, which have been shown to sink quickly to the seafloor and deliver pulses of carbon to benthic ecosystems. Thus, giant larvaceans can contribute to the vertical flux of microplastics through the rapid sinking of fecal pellets and discarded houses. Larvaceans, and potentially other abundant pelagic filter feeders, may thus comprise a novel biological transport mechanism delivering microplastics from surface waters, through the water column, and to the seafloor. Our findings necessitate the development of tools and sampling methodologies to quantify concentrations and identify environmental microplastics throughout the water column.
INTRODUCTION
Globally, plastic waste is now a pervasive feature of nearly all marine environments, ranging from coastal bays and estuaries to large oceanic gyres and down to the abyssal seafloor (1–3). In the absence of changes to current waste management practices, the annual amount of plastic waste available to enter the ocean from land is predicted to rise to 250 million metric tons by 2025 (4). Documented ecosystem impacts from this widespread contamination by plastic debris range widely from physical hazards and digestive blockage from ingestion by marine life to ecotoxicological effects resulting from the trophic transfer of plastic-related contaminants through marine food webs (5–7). Of the diverse marine habitats from which plastic debris have been documented, the distribution and fate of plastics in open ocean ecosystems are the most poorly known. Offshore marine waters comprise the largest living space on the planet, and the diversity of ecosystem inhabitants form complex food webs of vast international economic importance. Within the open ocean, a spectrum of plastics has been globally documented from surface waters (2, 8) and, more recently, in the seafloor sediments of deep-ocean basins (9, 10). Thus, along with sinking organic matter that fuels most of the deep-sea ecosystems, there is a poorly understood but potentially highly dynamic plastic debris field [including negatively buoyant materials (11)] sinking through the ocean’s mid-waters, for which ingestion and vertical transport processes are virtually unknown (3).
Microplastics (defined as particles <5 mm in diameter) are the most numerically abundant size range of particles within marine plastic debris fields (2). Despite their pervasiveness, we lack a comprehensive understanding of environmental microplastic distributions, their sources and sinks, physical and biological transport mechanisms, and the associated organismal and human health hazards. Microplastics arguably exert the largest ecosystem-scale pollution due to their small sizes, which allow for ingestion by pelagic organisms ranging from zooplankton (12), micronekton (13), and even large predatory fishes (14). However, the biological impacts on organismal feeding, growth, reproduction, and mortality are poorly known, in part because there is a lack of in situ data on the ingestion of microplastics and subsequent digestive processing (5). Nevertheless, there is evidence that the chemical burdens associated with microplastics present ecotoxic hazards to marine animals (7, 15). Here, to better understand the ability of midwater consumers to ingest and process microplastics, we conducted in situ feeding experiments with abundant filter-feeding larvaceans, Bathochordaeus stygius, from the open ocean ecosystem of Monterey Bay, California.
Larvaceans, or appendicularians, are active filter feeders that are circumglobal components of marine zooplankton assemblages (16), whose abundances are often second only to those of copepods (17). They are basal chordates whose morphology consists of a trunk (or head) and a tail (Fig. 1). Larvaceans secrete complex mucus filters that form a “house” in which the animal lives (18). By beating their tails to drive a feeding current through their mucus houses, larvaceans are able to concentrate food particles and other media suspended in the water column. Giant larvaceans of the genus Bathochordaeus are generally an order of magnitude larger than most other larvacean species, building mucus houses in excess of 1 m in diameter (19). Because of their abundance and highest known filtration rates by any midwater filter feeder (average of 42.9 liters hour−1; maximum of 76.2 liters hour−1], giant larvaceans in Monterey Bay have the capacity to completely filter their principal depth range in less than 2 weeks (20). Discarded Bathochordaeus houses sink rapidly to the seafloor and contribute significantly to the vertical transport of carbon to the deep sea (21).
Fig. 1. Experiments were conducted on board R/V Western Flyer using DeepPIV’s particle injector deployed from ROV Doc Ricketts.
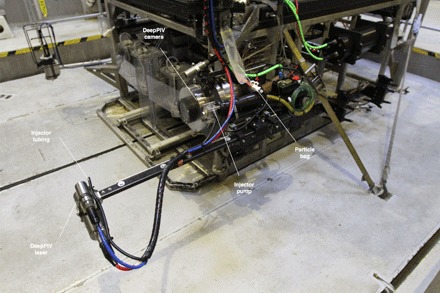
We conducted in situ feeding studies of giant larvaceans using a modified dye injector pump developed with the DeepPIV hardware (20). A peristaltic injector pump emptied a bag filled with microplastic particles and dispensed particle-rich fluid through the tubing nearby an animal.
Giant larvaceans are ideal candidates to investigate microplastic ingestion because their feeding filters exclude and subsequently concentrate particles of the same size range as microplastics (<5 mm), and ingestion of microplastics can be observed directly within the animal’s transparent trunk. Midwater larvaceans are also highly abundant and, like other filter feeders, generate currents to ingest particles of organic matter from the water column. Because of their effective filtration rates [up to ~80 liters hour−1 (20)] in combination with their status as important prey items (22), giant larvaceans could be a strong vector for microplastics into marine food webs. We used a remotely operated vehicle (ROV) outfitted with novel instrumentation, Deep Particle Image Velocimetry (DeepPIV; Fig. 1) (20) to make observations of giant larvaceans feeding on experimentally released microplastics with sizes ranging from 10 to 600 μm in diameter (see Materials and Methods). We evaluated (i) the potential for this filter feeder to ingest microplastics, (ii) the size range of ingested particles, and (iii) whether microplastics can be incorporated into the mucus house and/or fecal pellets, enabling the rapid removal of microplastics from near-surface waters and subsequent deposition on the seafloor.
RESULTS
Microplastic particles were dispensed on a total of 25 B. stygius individuals; particles were observed to enter the inner filters of 11 of these individuals. At-sea conditions limited our ability to conduct sustained ROV observations on many of the animals. Of these 11 individuals, microplastics were subsequently processed into the guts of 6 specimens. Some particles were dislodged by water flow from the internal surfaces of the inner filter and passed through the buccal tube and into the gut of the larvacean (Fig. 2 and movie S1). No particles were observed to exit from this pathway. Of the five individuals subsequently collected by the ROV, ship- and shore-based microscopy confirmed the presence of microplastics in the guts and/or fecal pellets of four specimens; one individual was lost during ROV recovery (Fig. 3 and table S1). All size classes of particles used in the feeding experiments were ingested (table S1). During and after passing of microplastic-rich fecal pellets, the collected specimens appeared to swim normally within their holding containers.
Fig. 2. During feeding experiments, microplastics were observed inside and attached to the inner house and inside the gut of giant larvaceans.
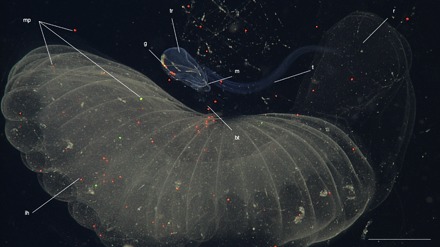
Microplastic particles in varying size ranges (from 10 to 600 μm) are represented by different colors (for example, red, yellow, green, and orange; see table S1 for specific particle sizes). Image corresponds to B. stygius specimen D5 from dive D870 (table S1). Scale bar, 2 cm. ih, inner house; bt, buccal tube; mp, microplastics; g, gut; tr, trunk; m, mouth; t, tail; r, ramp of inner house.
Fig. 3. Collected giant larvaceans were maintained in a cold room, and after 12 hours, animals and fecal pellets were imaged under a microscope to determine microplastic particle size.
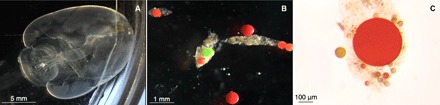
(A) After the 12-hour period, the guts of intact individuals were completely evacuated. (B) Microplastic particles were incorporated into the fecal pellets, where each color (for example, red, orange, yellow, and green) corresponds to specific size classes of particles shown at lower (B) and higher (C) magnifications. (See table S1 for specific particle sizes). The fecal pellet in (C) can be seen in the upper left corner of (B). Images shown here correspond to B. stygius specimen D5 from dive D870 (table S1).
Microplastic particles trapped within the collapsed filters of discarded Bathochordaeus houses would travel quickly to the seafloor, based on published sinking rates of about 800 m day−1 (21). We also measured the sinking rates of Bathochordaeus fecal pellets from specimens not used in the feeding experiments (and thus not containing microplastics) and found that these pellets descend at an average rate of 300 m day−1 (see Materials and Methods and table S2). These relatively fast sinking rates (23) reduce the likelihood of interception, consumption, or degradation during their fall to the seafloor. Despite the near-neutral buoyancy of particles selected for this feeding study, the collected microplastic-rich mucus houses and fecal pellets were negatively buoyant in their holding containers. While these observations support the assumption that microplastic-rich structures sink, the rates of deposition are expected to vary because sinking rate inherently depends on the density of the ballasting material (24).
DISCUSSION
Our findings show that giant larvaceans can ingest and package microplastics into sinking aggregates. Arguments that could limit the significance of these results include the following: (i) Microplastics may be disproportionately constrained to the top of the water column, above the principal depth range of Bathochordaeus (100 to 300 m) (20), and (ii) giant larvaceans may be able to selectively reject microplastics at concentrations found within their environment. However, microplastics have been reported from different subsurface marine habitats, such as abyssal ocean sediments (10) and the upper water column (25, 26), directly suggesting a dynamic microplastic particle field sinking through the water column. More measurements of concentrations and distributions of microplastics across the water column are needed. With regard to particle rejection, studies on smaller, near-surface larvacean species using fluorescent plastic and latex particles may extend our findings of microplastic uptake to the oikopleurids Oikopleura dioica (27–29), O. vanhoeffeni (30), and Stegasoma magnum (27) and to the fritillarid Fritillaria borealis (29).
Oikopleurids (including Bathochordaeus) are among the most abundant filter feeders found in the ocean (17); oikopleurids and fritillarids occur at depths extending from the surface to 3500 m (31, 32). Although very little is known about subsurface microplastic distributions, filter-feeding larvaceans can be found anywhere in the oceanic water column where microplastics are expected to occur. For example, larvaceans have been reported as important components of zooplankton assemblages in the subtropical and subarctic North Pacific regions (33), oceanic areas that encompass microplastic convergence zones associated with the North Pacific Subtropical Gyre (2). Larvaceans are known to be able to actively reject particles during ingestion and to have the ability to differentiate particles by size, nutrient concentration, and the presence of toxins (34). Given these abilities, larvaceans would be expected to differentiate nutrient-rich prey items from nutrient-poor microplastics. However, laboratory feeding studies on O. dioica found no difference between grazing rates on plastic beads and natural phytoplankton (28), which is consistent with our in situ ingestion observations of B. stygius.
Our results present a novel biological transport vector that could effectively move large amounts of microplastics from near-surface waters into the deep sea. While the overall importance of this pathway is dependent upon relatively unmeasured environmental concentrations and characterizations of microplastics across the water column [for example, abundance, size range, and microplastic material properties including density (35)], our results suggest multiple, interacting areas that require further study. Larvaceans have been shown to be the primary prey for many planktonic carnivores and larval fish (22) and could thus serve as an important trophic node for the transfer of microplastics through marine food webs. In addition, the discarded houses of larvaceans are widely consumed by a diversity of mesopelagic and bathypelagic animals, as well as benthic organisms on the seafloor (36). The same is likely to be true for larvacean fecal pellets [for example, see study of Turner (23)]. A number of other highly abundant pelagic filter feeders and suspension feeders, including salps, doliolids, and pyrosomes (35), are potential consumers of microplastics and could also transfer plastics through food webs. Little is known about whether these widespread animal groups ingest microplastics in situ, although laboratory feeding studies on salps have demonstrated plastic ingestion (37). To comprehensively evaluate the overall impacts of microplastics on marine food webs and nutrient cycling in the ocean, targeted efforts are required to gather data on the rates and concentrations at which microplastics are aggregated by larvaceans and other pelagic filter-feeders.
MATERIALS AND METHODS
Preparation of microplastics for in situ feeding studies
Polyethylene microspheres, or microplastic particles (Cospheric LLC), were selected based on particle density (closely matching the density of seawater or 1.027 g/cm3) and size range, while minimizing overlap of coloration to distinguish between particle size classes during feeding experiments. Given these constraints, we selected red, orange, yellow, and green microplastic particles that ranged in size from 10 to 600 μm in diameter (table S1). All particles but the largest size class (red; 500 to 600 μm in diameter) had fluorescent surface coatings to enhance visibility for observations during experiments. Microplastics were prepared using a protocol that uses Tween 80 biocompatible surfactant to ensure separation of particles once in solution (www.cospheric.com/tween_solutions_density_marker_beads.htm). To minimize any adverse behavioral responses to the surfactant, the Tween solution was replaced with seawater at the end of the protocol, which did not have any visible effect on particle separation. Before each ROV dive, an intravenous bladder with a volume of 1 liter was filled with a mixture of 200 ml of microalgae (cultured Nannochloropsis sp.), 1.25 g of prepared microplastics (0.25 g of each size class), and 800 ml of filtered seawater. The addition of microalgae to suspended microplastics is consistent with previous studies of larvacean filter feeding (28, 29). Microplastic concentrations within the bladder were approximately 1.25 g/cm3 for all feeding experiments.
Microplastic dispersal using modified DeepPIV dye injector
In situ feeding experiments were enabled by the deployment of DeepPIV (20) from the port manipulator on ROV Doc Ricketts. DeepPIV is an instrument that allows for the in situ visualization and quantification of small-scale fluid motion (Fig. 1). In addition to camera and laser housings, DeepPIV was equipped with a peristaltic pump (Cole Parmer), whose tubing diameter allowed for the passage of particles greater than 1 mm in size. An intravenous bladder filled with the microplastic mixture was attached to the injector such that microplastic particle–rich seawater was released from the injector tubing once the peristaltic pump was activated (fig. S1).
In situ feeding experiments with giant larvaceans
Feeding experiments with B. stygius were conducted in Monterey Bay, California, in June 2016 using ROV Doc Ricketts and DeepPIV and occurred between depths of 200 and 400 m. During in situ feeding experiments, an ROV pilot positioned the vehicle such that the injector tubing was adjacent to the larvacean inner house. Particle-rich fluid was dispensed into the water column for 1- to 5-s intervals depending on the quantity of particles released (fig. S1, 00:00:00). After particle release, the ROV was positioned away from the animal such that the hydrodynamic signature of the vehicle did not disturb larvacean feeding behavior. Sustained observations of a feeding larvacean would continue until microplastic particles could be visually confirmed inside the gut of the animal (fig. S1, 00:01:50) or until proper positioning between the ROV and surface vessel could no longer be achieved, whichever was longer. At the completion of the experiment, the animal was collected in a detritus sampler (fig. S1, 00:10:15), stowed, and brought to the surface at the end of the ROV dive. Once at the surface, animals were transferred from the samplers into separate containers and kept overnight in a cold room. Over the ensuing 12 hours, those specimens produced fecal pellets that were collected and imaged using ship-based microscopy to confirm the presence of microplastics (Fig. 3, A and B). Animals and fecal pellets were then preserved in formalin and brought to the shore for further microscope imaging to quantify the size distribution of particles ingested (Fig. 3C and table S1). Movie S1 shows an in situ feeding experiment, which includes particle injection, microplastic ingestion, and collection of giant larvacean specimen D5 during dive D870 (table S1).
Sinking velocities of giant larvacean fecal pellets
Seven specimens of Bathochordaeus spp. were collected in August 2016 using ROV Ventana from depths between 100 and 200 m. Thirteen fecal pellets (derived from food captured by the Bathochordaeus specimens before capture and then generated by those specimens) were photographed, measured, and used to determine sinking rates (table S2). Giant larvacean specimens were kept at 9°C in an environmental chamber, and seawater from each unique collection was transferred to a chilled, glass graduated cylinder 50 mm in diameter. Sinking experiments commenced once water movement in the graduated cylinder ceased. Fecal pellets were introduced individually into the graduated cylinder using a large-bore pipette and timed as they settled through the 243-mm column. Fecal pellets were video-recorded as they sank and subsequently collected and preserved in 5% formalin.
Acknowledgments
We are grateful for the engineering contributions made by D. Graves, C. Kecy, D. Klimov, J. Erickson, and the technical staff of the Monterey Bay Aquarium Research Institute (MBARI) to the development of DeepPIV. We would also like to thank D. Li and K. Uhlinger for providing the microalgae cultures. The incredible contributions of the crew of R/V Western Flyer and the pilots of ROV Doc Ricketts made these observations possible. Funding: This work was supported by the David and Lucile Packard Foundation. C.A.C. was partially supported by the Monterey Bay Aquarium. Author contributions: K.K. and C.A.C. wrote the manuscript. K.K., C.A.C., A.D.S., R.E.S., and B.H.R. contributed to and edited the manuscript. K.K., C.A.C., and R.E.S. conducted the experiments. K.K., A.D.S., and B.H.R. designed the experiments. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. In addition, the data reported in this paper can be searched using MBARI’s Video Annotation and Reference System database (www.mbari.org/products/research-software/video-annotation-and-reference-system-vars).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1700715/DC1
fig. S1. In situ feeding experiments with giant larvaceans involved quantifying filtration rates, particle injections, feeding observations, collection of animals, and imaging of animal guts and subsequent fecal pellets.
table S1. Summary of in situ feeding results of B. stygius individuals collected during dive D870 on R/V Western Flyer.
table S2. Summary of fecal pellet sinking results for giant larvaceans collected with ROV Ventana.
movie S1. In situ feeding experiment using microplastic particles with a giant larvacean B. stygius (individual D5) on dive D870 in June 2016.
REFERENCES AND NOTES
- 1.Schlining K., von Thun S., Kuhnz L., Schlining B., Lundsten L., Jacobsen Stout N., Chaney L., Connor J., Debris in the deep: Using a 22-year video annotation database to survey marine litter in Monterey Canyon, central California, USA. Deep Sea Res. Part I 79, 96–105 (2013). [Google Scholar]
- 2.Eriksen M., Lebreton L. C. M., Carson H. S., Thiel M., Moore C. J., Borerro J. C., Galgani F., Ryan P. G., Reisser J., Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLOS ONE 9, e111913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W. C., Tse H. F., Fok L., Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 566–567, 333–349 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Jambeck J. R., Geyer R., Wilcox C., Siegler T. R., Perryman M., Andrady A., Narayan R., Law K. L., Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Wright S. L., Thompson R. C., Galloway T. S., The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 178, 483–492 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Avio C. G., Gorbi S., Regoli F., Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Rochman C. M., Hoh E., Kurobe T., Teh S. J., Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 3263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisser J., Slat B., Noble K., du Plessis K., Epp M., Proietti M., de Sonneville J., Becker T., Pattiaratchi C., The vertical distribution of buoyant plastics at sea: An observational study in the North Atlantic Gyre. Biogeosciences 12, 1249–1256 (2015). [Google Scholar]
- 9.Van Cauwenberghe L., Vanreusel A., Mees J., Janssen C., Microplastic pollution in deep-sea sediments. Environ. Pollut. 182, 495–499 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Woodall L. C., Sanchez-Vidal A., Canals M., Paterson G. L. J., Coppock R., Sleight V., Calafat A., Rogers A. D., Narayanaswamy B. E., Thompson R. C., The deep sea is a major sink for microplastic debris. Roy. Soc. Open Sci. 1, 140317–140317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrady A. L., Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T. S., Microplastic ingestion by zooplankton. Environ. Sci. Technol. 47, 6646–6655 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Davison P., Asch R. G., Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol. Prog. Ser. 432, 173–180 (2011). [Google Scholar]
- 14.Choy C. A., Drazen J. C., Plastic for dinner? Observations of frequent debris ingestion by pelagic predatory fishes from the central North Pacific. Mar. Ecol. Prog. Ser. 485, 155–163 (2013). [Google Scholar]
- 15.Teuten E. L., Saquing J. M., Knappe D. R. U., Barlaz M. A., Jonsson S., Björn A., Rowland S. J., Thompson R. C., Galloway T. S., Yamashita R., Ochi D., Watanuki Y., Moore C., Viet P. H., Tana T. S., Prudente M., Boonyatumanond R., Zakaria M. P., Akkhavong K., Ogata Y., Hirai H., Iwasa S., Mizukawa K., Hagino Y., Imamura A., Saha M., Takada H., Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 364, 2027–2045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R. Fenaux, Q. Bone, D. Deibel, in The Biology of Pelagic Tunicates, Q. Bone, Ed. (Oxford Univ. Press, 1998), pp. 251–264. [Google Scholar]
- 17.Hopcroft R. R., Roff J. C., Production of tropical larvaceans in Kingston Harbour, Jamaica: Are we ignoring an important secondary producer? J. Plankton Res. 20, 557–569 (1998). [Google Scholar]
- 18.Alldredge A. L., House morphology and mechanisms of feeding in the Oikopleuridae (Tunicata, Appendicularia). J. Zool. 181, 175–188 (1977). [Google Scholar]
- 19.Hamner W. M., Robison B. H., In situ observations of giant appendicularians in Monterey Bay. Deep Sea Res. Part A 39, 1299–1313 (1992). [Google Scholar]
- 20.Katija K., Sherlock R. E., Sherman A. D., Robison B. H., New technology reveals the role of giant larvaceans in oceanic carbon cycling. Sci. Adv. 3, e1602374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robison B. H., Reisenbichler K. R., Sherlock R. E., Giant larvacean houses: Rapid carbon transport to the deep sea floor. Science 308, 1609–1611 (2005). [DOI] [PubMed] [Google Scholar]
- 22.J. E. Purcell, M. V. Sturdevant, C. P. Galt, in Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularians, G. Gorsky, M. J. Youngbluth, D. Deibel, Eds. (Éditions Scientifiques, 2005), pp. 359–435. [Google Scholar]
- 23.Turner J. T., Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog. Oceanogr. 130, 205–248 (2015). [Google Scholar]
- 24.Lombard F., Guidi L., Kiørboe T., Effect of type and concentration of ballasting particles on sinking rate of marine snow produced by the appendicularian Oikopleura dioica. PLOS ONE 8, e75676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desforges J.-P. W., Galbraith M., Dangerfield N., Ross P. S., Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 79, 94–99 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Kukulka T., Proskurowski G., Morét-Ferguson S., Meyer D. W., Law K. L., The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 39, L07601 (2012). [Google Scholar]
- 27.Alldredge A. L., The impact of appendicularian grazing on natural food concentrations in situ. Limnol. Oceanogr. 26, 247–257 (1981). [Google Scholar]
- 28.Bedo A. W., Acuña J. L., Robins D., Harris R. P., Grazing in the micron and the sub-micron particle size range: The case of Oikopleura dioica (Appendicularia). Bull. Mar. Sci. 53, 2–14 (1993). [Google Scholar]
- 29.Fernández D., López-Urrutia Á., Fernández A., Acuña J. L., Harris R. P., Retention efficiency of 0.2 to 6 μm particles by the appendicularians Oikopleura dioica and Fritillaria borealis. Mar. Ecol. Prog. Ser. 266, 89–101 (2004). [Google Scholar]
- 30.Deibel D., Filter feeding by Oikopleura vanhoeffeni: Grazing impact on suspended particles in cold ocean waters. Mar. Biol. 99, 177–186 (1988). [Google Scholar]
- 31.R. R. Hopcroft, in Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularians, G. Gorsky, M. J. Youngbluth, D. Deibel, Eds. (Contemporary Publishing International, 2005), pp. 45–57. [Google Scholar]
- 32.Robison B. H., Sherlock R. E., Reisenbichler K. R., The bathypelagic community of Monterey Canyon. Deep Sea Res. Part II 57, 1551–1556 (2010). [Google Scholar]
- 33.Steinberg D. K., Cope J. S., Wilson S. E., Kobari T., A comparison of mesopelagic mesozooplankton community structure in the subtropical and subarctic North Pacific Ocean. Deep Sea Res. Part II 55, 1615–1635 (2008). [Google Scholar]
- 34.Lombard F., Selander E., Kiørboe T., Active prey rejection in the filter-feeding appendicularian Oikopleura dioica. Limnol. Oceanogr. 56, 1504–1512 (2011). [Google Scholar]
- 35.Riisgård H. U., Larsen P. S., Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 418, 255–293 (2010). [Google Scholar]
- 36.Alldredge A. L., Discarded appendicularian houses as sources of food, surface habitats, and particulate organic matter in planktonic environments. Limnol. Oceanogr. 21, 14–24 (1976). [Google Scholar]
- 37.Sutherland K. R., Madin L. P., Stocker R., Filtration of submicrometer particles by pelagic tunicates. Proc. Natl. Acad. Sci. U.S.A. 107, 15129–15134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1700715/DC1
fig. S1. In situ feeding experiments with giant larvaceans involved quantifying filtration rates, particle injections, feeding observations, collection of animals, and imaging of animal guts and subsequent fecal pellets.
table S1. Summary of in situ feeding results of B. stygius individuals collected during dive D870 on R/V Western Flyer.
table S2. Summary of fecal pellet sinking results for giant larvaceans collected with ROV Ventana.
movie S1. In situ feeding experiment using microplastic particles with a giant larvacean B. stygius (individual D5) on dive D870 in June 2016.


