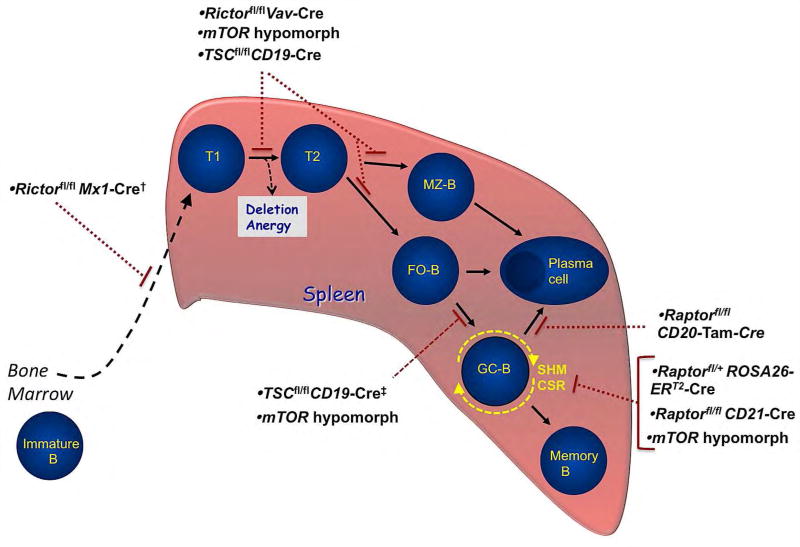
Figure 5. Model of peripheral B cell development in the spleen with associated blocks in mTORC1 and mTORC2 mutants.
Immature B cells migrate from the bone marrow and fetal liver to the spleen and proceed through transitional stages Tl and T2, where they continue to be tested for self-reactivity before differentiating into mature marginal zone (MZ-B) or follicular (FO) B cells. Upon encounter with specific antigen and with the requisite T-cell-derived co-stimulation, activated follicular B cells may differentiate into either short-lived antibody secreting plasma cells or into germinal center B cells (GC-B). GC-B cells undergo clonal expansion, somatic hypermutation, and class-switch recombination before terminally differentiating into long-lived antibody-secreting plasma cells or memory B cells. MZ-B cells are also capable of differentiating into plasma cells. Gene-targeted deletion of specific mTORC1 and/or mTORC2 signaling components in mice disrupt splenic B cell development at the stages shown.
†The specific developmental block in Rictorfl/flMx1-Cre mice has not been characterized. These mice develop a normal immature B cell populations in the bone marrow, but have reduced mature B cells.
‡One study showed TSC1fl/flCD19-Cre mice have a defect in GC-B cell formation, while the other study showed no impairment.