Figure 1. Chronic activation of the Ire1/Xbp1 pathway reduces Aβ42 levels, but causes age-dependent behavioral deficits, in a Drosophila model of chronic ER proteinopathy.
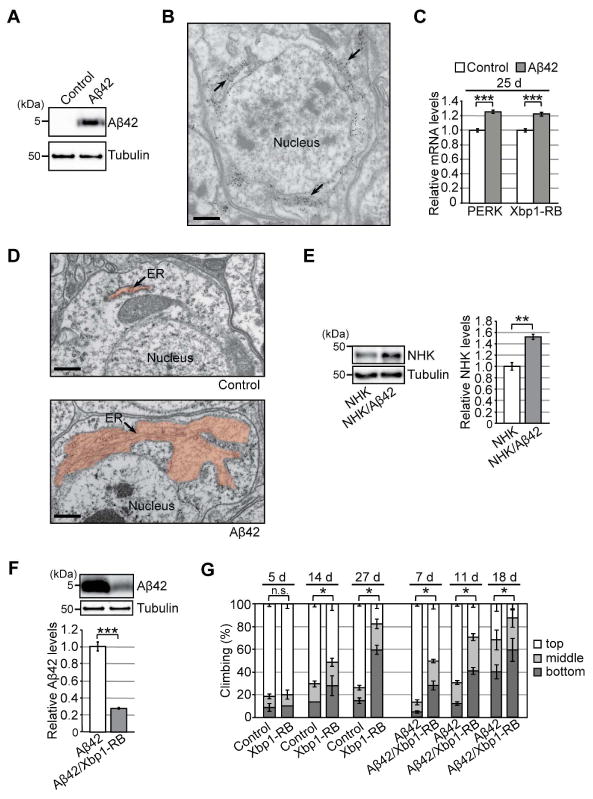
(A) Expression of Aβ42 peptides in fly brains. Heads from flies carrying the elav-GAL4 driver alone (Control) or the elav-GAL4 driver and UAS-Aβ42 (Aβ42) were subjected to western blotting using an anti-Aβ antibody. Tubulin was used as the loading control. (B) Immuno-EM analysis of Aβ42 distribution in fly brain neurons. Gold particles were detected in the ER. Scale bar: 0.5 μm. (C) mRNA levels of PERK and Xbp1-RB were increased in Aβ42 fly brains, as determined by qRT-PCR. Mean ± SEM, n = 4, ***p < 0.001 by Student's t-test. (D) Abnormal enlargement of the ER was observed in neurons expressing Aβ42 (indicated by arrow). Scale bars: 0.5 μm. (E) Levels of exogenously expressed null Hong Kong α1-antitrypsin (NHK) protein were elevated in Aβ42 fly brains. Mean ± SEM, n = 3–4, **p < 0.01 by Student's t-test. (F) Overexpression of Xbp1-RB reduced Aβ42 levels in fly brains. Mean ± SEM, n = 4, ***p < 0.001 by Student's t-test. (G) Overexpression of Xbp1-RB in neurons caused age-dependent locomotor deficits and worsened behavioral deficits in Aβ42 flies. Average percentages of flies that climbed to the top (white) or middle (light gray), or stayed at the bottom (dark gray), of the vials. Ages (d, days after eclosion) are indicated on the top of the graph. Percentages of flies that stayed at the bottom were subjected to statistical analyses. Mean ± SD, n = 5, *p < 0.05 by Mann–Whitney U test. n.s.: not significant. See also Figure S1 and Table S5.
