Figure 3. Neuronal knockdown of Sin3A increases mRNA levels of Drosophila EDEMs (dEDEMs), and upregulation of these factors is sufficient to reduce Aβ42 levels in brain.
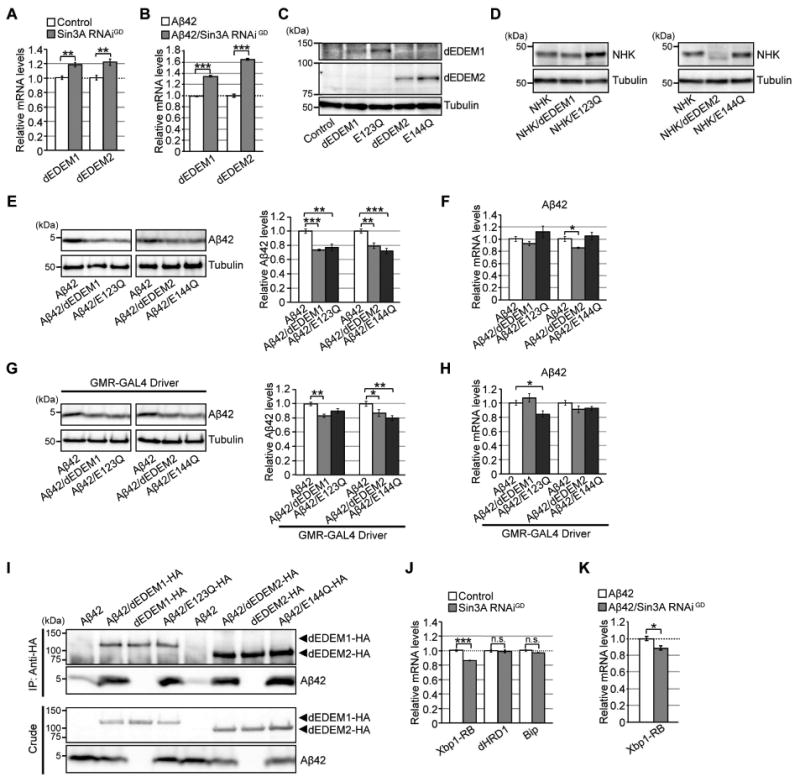
(A–B) Neuronal knockdown of Sin3A increased mRNA levels of dEDEM1 or dEDEM2 in both Control and Aβ42 fly brains. Mean ± SEM, n = 4, **p < 0.01 and ***p < 0.001 by Student's t-test. (C) Expression of dEDEM proteins in transgenic fly brains. Heads of flies expressing dEDEMs were analyzed by western blotting with anti-HA antibody. (D) Mannosidase activities of dEDEMs were critical for degradation of misfolded glycoprotein NHK in fly brains. Heads of flies expressing NHK alone or co-expressing NHK and dEDEMs were analyzed by western blotting. (E, G) Overexpression of dEDEMs reduced Aβ42 levels in neurons (E) and eyes (G). Heads of flies expressing Aβ42 alone or co-expressing Aβ42 and dEDEMs were analyzed by western blotting with anti-Aβ antibody. Mean ± SEM, n = 3–4, *p < 0.05, **p < 0.01, and ***p < 0.001 by Student's t-test. (F, H) Overexpression of dEDEMs had minimal effects on Aβ42 mRNA levels in neurons (F) and eyes (H), as determined by qRT-PCR. Mean ± SEM, n = 4, *p < 0.05 by Student's t-test. (I) dEDEMs co-immunoprecipitated with Aβ42 peptides. Drosophila S2 cells were transiently transfected with HA epitope-tagged dEDEMs-HA, Aβ42 and dEDEMs-HA, or Aβ42 alone. Cell lysates were subjected to immunoprecipitation with anti-HA antibody, followed by western blotting with anti-Aβ or anti-HA antibody. Top two panels show immunoprecipitates, and bottom two panels show crude lysates. (J–K) Neuronal knockdown of Sin3A did not increase mRNA levels of Xbp1-RB, dHRD1, or BiP in control or Aβ42 fly brains, as determined by qRT-PCR. Mean ± SEM, n = 4, *p < 0.05 and ***p < 0.001. n.s.: not significant (Student's t-test). See also Figure S3 and Tables S2 and S5.
