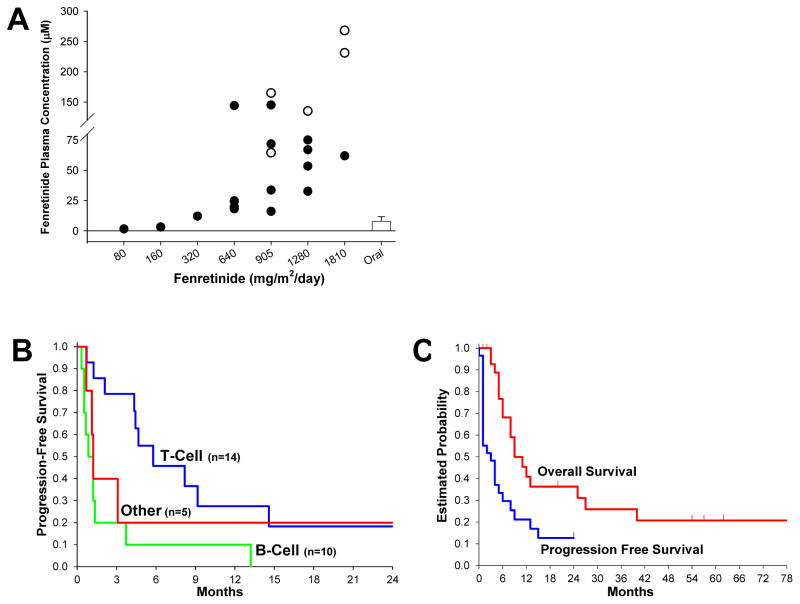
Figure 1.
(A) End-infusion fenretinide plasma levels for dosing cohorts (+120 hours). Closed circles represent patients completing infusion course. Open circles represent last plasma sample of patients with infusions stopped due to hypertriglyceridemia. Bar represents reported achievable plasma levels using oral capsule dosed on seven-day schedule. (B) Progression Free Survival (PFS) of evaluable patients by tumor type; peripheral T-Cell (n=14), median 5.7 months (95% CI, 2.1 to 14.6); mature B-Cell lymphoma or leukemia (n=10), median 0.8 months (95% CI, 0.5 to 1.3) months; Other hematologic malignancy (n=5), median 1.2 months (95% CI, 0.6 to not available); difference in PFS between T-cell and B-cell, P < 0.011 (log-rank test). (C) Overall Survival (OS), median (95% CI): 9.3 (5.5, 25.4) months, and Progression Free Survival (PFS), median (95% CI): 3.0 (1.2, 5.7) months, for all patients (n = 29) over all dose levels.