Abstract
Clinical and experimental data suggest that salicylic acid (SA) is tumor preventive and NO has a multitude of effects on tumor biology. Therefore, firstly, the aim of our study is to explore the important role of SA in apoptotic induction of liver cancer cells. Secondly, we investigate whether SA mediates the anti-tumor effects by NO signaling pathway. The liver cancer cell line was treated with different concentrations of SA. Cell proliferation was tested using MTS assay and cell apoptosis was assessed by flow cytometry. NO content and NOS activities were measured by biochemical assay. The anti- or pro-apoptotic regulator gene expressions were analyzed by real-time PCR. Our data illustrated that high concentration of SA significantly inhibited liver cancer cell proliferation accompanied by apoptosis induction. In addition, SA led to the release of NO and the increase of NOS activities in above process. Importantly, SA up-regulated a series of apoptosis-related gene expression and reduced the mRNA level of HMGB1. Meanwhile, we also found that NOS inhibitor L-NAME and NO scavenger cPTIO attenuated the above SA-induced effects. Thus, we provided the evidence that SA exerted anti-tumor effects in liver cancer cell in part mediated by the NO pathway.
Keywords: Salicylic acid, Apoptotic inducible effect, Hepatoma cell line, Nitric oxide
Introduction
Recently, several decades of research have provided considerable evidence for the prevention role of aspirin for patients with cancer (Annemijn 2012; Menamin et al. 2015; Giampieri et al. 2016; Elwood et al. 2016). Interestingly, salicylic acid, as one of the aspirin metabolite in vivo (Paterson et al. 2001; Klessig 2016), could induce apoptosis selectively in malignant cells in vitro (Chung et al. 2003; Elder 1996; Klampfer 1999; Zitta et al. 2012) and showed anti-tumor effects in vivo (Elwood 2009). However, the mechanistic terms have not been sufficiently explained. Therefore, there is a pressing need to understand the protective mechanisms of salicylic acid against cancer.
Nitric oxide (NO), which is synthesized by three NOS isoforms in vivo, neuronal (nNOS/NOS1), endothelial (eNOS/NOS3), and inducible (iNOS/NOS2) (Nathan 1997; Klotz et al. 1998), has been invoked in nearly every normal (Palmer et al. 1987) and pathological condition associated with human physiology (Moncada et al. 1991; Xu and Liu 1998). In tumor biology, NO has been characterized and implicated to play regulatory roles in various processes of carcinogenesis, including tumor initiation (Puglisi et al. 2015), growth (Jenkins et al. 1995; Reveneau etal.1999; Ambs et al. 1998), invasion and metastasis (Vakkala et al. 2000), inflammation (Kanwar et al. 2009), and modulation of therapeutic responses (Wink et al. 1997; Hagos et al. 2007). So far, no link between NO generation and salicylic acid anti-tumor effects has been established.
High-mobility group box chromosomal protein 1 (HMGB1), an evolutionarily conserved protein, has been demonstrated to play a potential role in anti-cancer therapy (Sims et al. 2010). HMGB1 could stimulate a number of proteins involved in proliferation of cancer cells and inhibits signals that control cell growth. In addition, HMGB1 were identified as SA targets, which modulate inflammatory responses (Choi et al. 2015). However, the role of HMGB1 in NO signal pathway during the process of SA-induced liver cell apoptosis has not been established.
Hepatocellular carcinoma (HCC) is the most common primary malignancy found in liver, with few effective therapeutic options for this severe disease (Llovet et al. 2003; Ferenci et al. 2010). Therefore, in this article, we investigated the ability of SA to prevent tumorigenetic in hepatoma cell line and evaluated the role of NO pathway in salicylic acid-dependent apoptosis induction. As the result, we will provide further information on establishing an impressive synergistic effect of SA.
Materials and methods
Materials
RPMI 1640 medium, FBS, penicillin-streptomycin, and trypsin-EDTA were purchased from HyClone (Thermo Scientific). TRIzol reagent was purchased from Invitrogen (Life Technology, USA). PrimeScript RT Reagent Kit and SYBR Premix EX Tag were purchased from TAKARA (TAKARA Biotechnology, Japan).
The compound 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO) was used as a specific NO scavenger. NG-nitro-l-arginine methylester hydrochloride (L-NAME) was used as the inhibitor of NOS. The other chemicals used, for example, salicylic acid, unless otherwise stated, were obtained from Sigma Chemicals (St. Louis, MO, USA).
Cell line and cell culture
Hepatoma cell line Bel-7402 (moderately differentiated cell line), HepG2 (well-differentiated cell line), and normal liver cell line LO2 were obtained from Shanghai Institute for Biological Sciences, Chinese Academy of Science (Shanghai, China) and were maintained in RPMI 1640 medium (HyClone) containing 10% (v/v) FBS (HyClone), 4 mM glutamine, 100 U penicillin/ml, and 100 mg streptomycin/ml at 37 °C and 5% CO2.
Treatments
Control stand for hepatoma cell line (Bel-7402 and HepG2) was grown under normal conditions. In all figures, “S” (100 μM, 500 μM, 1 mM, 5 mM, and 10 mM) stands for hepatoma cell line cells that were incubated at the suitable densities in the presence of SA (100 μM, 500 μM, 1 mM, 5 mM, and 10 mM) for indicated hours. Among them, 5 mM SA was chosen as the suitable treatment because of its inhibition rate IC50 <50%. The further experimental design thus consisted of the following treatment groups: NOS inhibitor L-NAME (0.1 mM) and NO scavenger cPTIO (0.1 mM) were used, respectively. (S5mM + L-NAME) and (S5mM + cPTIO) stand for 5 mM concentrations of SA treatment in the presence of L-NAME and cPTIO, respectively. In all experiments, assays were performed in duplicate and the above solutions were replaced each day. All tests were carried out in at least three independent sets of experiments with similar results.
Evaluation of cell viability by MTS assay
Cell viability was assessed by the MTS assay. Hepatoma cells were seeded into 96-well plates at a density of 2 × 103 cells/well in 0.1 ml medium with different treatments for 24 and 48 h. After that, all media were aspirated and gently washed with 1× PBS twice. Then 100 μl of RPMI 1640 medium was mixed with 20 μl of MTS solution per well. The mixture was placed in an incubator at 37 °C and 5% CO2 for 1 h. Absorbance was measured at 490 nm (the absorbance on CTL was used to subtract the background absorbance). All experiments were performed in triplicate.
Apoptosis analyses
Hepatoma cells were plated (1 × 105 per well) in a six-well plate and exposed to different treatments. After 24 h, apoptosis was detected using the Annexin V-FITC/PI apoptosis detection kit. Briefly, hepatoma cells were washed twice with ice-cold PBS and re-suspended in binding buffer. Cell suspensions were stained with 5 μl of Annexin V-FITC conjugate and 10 μl of PI. Samples were analyzed by FACS Calibur (BD FACS Calibur TM, San Jose, CA) and FlowJo (Tree Star, San Carlos, CA) analysis software.
The measurement of NO
The transient and volatile nature of NO makes it unsuitable for most convenient detection methods; however, two stable breakdown products, nitrate (NO3−) and nitrite (NO2−), can be easily detected by photometric means. In this experiment, the Griess method based on the chemical diazotization reaction was adopted to detect NO, which uses sulfanilamide and N-(1-napthyl)ethylenediamine dihydrochloride (NED) under acidic (phosphoric acid) conditions.
After cells were incubated according to the aforementioned grouping, 50 μl culture solutions of each well were collected and put into the counterpart well of another plate. Then NO production in cells was measured according to the indication on the NO assay kit (Beyotime, China). The data were analyzed with Statistical Program for Social Sciences.
NO synthase activity determination
Total and inducible NO synthase activity was measured by monitoring the NO concentration using a nitric oxide synthase assay kit (Beyotime Corporation). Cells were cultured with different treatments for 24 h, and the cultured cells were collected and transferred to a 96-well plate. Assay buffer, the l-arginine substrate, and the tracer DAF-FMDA (3-amino-4-aminomethyl-20, 70-difluorescein diacetate) were added, followed by addition of 0.1 mM NADPH to the reaction mixtures to initiate the reactions. The plate was incubated at 37 °C for 30 min, centrifuged, and washed three times in PBS. After keeping at room temperature for 20 min, the fluorescence of the plate was measured by a Bio-Rad iMark instrument (ex = 495 nm, em = 515 nm). Instructions from the manufacturer were followed strictly, and each compound was tested in triplicate; the value given is an average of three determinations.
RNA extraction and real-time PCR analysis
Briefly, total RNA was isolated from cells with TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with PrimeScript RT Reagent Kit according to the manufacturer’s instructions. The obtained cDNA was used to determine the mRNA amount of inducible nitric oxide synthase (NOS2), endothelial nitric oxide synthase (NOS3), BCL2-Like11 (BCL2L11), BCL2-Like13 (BCL2L13), BCL2-Like14 (BCL2L14), Caspase 9 (CASP9), and HMGB1 by real-time PCR with Taq DNA polymerase (TAKARA). GAPDH was used as an internal control. The Ct value of each cell was recorded, and the data were analyzed by the comparative Delta-delta Ct method. The primers used for amplification are listed as follows:
| Gene | Primer |
| 1.NOS2 | Forward: 5′-TTCAGTATCACAACCTCAGCAAG-3′ Reverse: 5′-TGGACCTGCAAGTTAAAATCCC-3′ |
| 2.NOS3 | Forward: 5′-TGATGGCGAAGCGAGTGAAG-3′ Reverse: 5′-ACTCATCCATACACAGGACCC-3′ |
| 3.BCL2L11 | Forward: 5′-TAAGTTCTGAGTGTGACCGAGA-3′ Reverse:5′- GCTCTGTCTGTAGGGAGGTAGG -3′ |
| 4.BCL2L13 | Forward: 5′-AGGACTATTCGGCAGAGTACAT-3′ Reverse: 5′-TGATTCCAGGGTATTCCTCCTC-3′ |
| 5.BCL2L14 | Forward: 5′-CAAAATCCTCGCCTACTACACC-3′ Reverse: 5′-GACTCATTTGCTGAACAATTCCC-3′ |
| 6.CASP9 | Forward: 5′-CTCAGACCAGAGATTCGCAAAC-3′ Reverse: 5′-GCATTTCCCCTCAAACTCTCAA-3′ |
| 7.HMGB1 | Forward: 5′-TCGCGAATTCATGGCAAAGGAG-3′ Reverse: 5′-GCTTTTATTCATCATCATCATC-3′ |
| 8.GAPDH | Forward: 5′-TGCACCACCAACTGCTTAGC-3′ Reverse: 5′-GGCATGGACTGTGGTCATGAG-3′ |
Statistical analysis
All data were analyzed by SPSS 16.0 software (SPSS Inc., Chicago, IL) and presented as mean ± SE from at least three independent experiments performed in triplicate. Different measurements were subjected to analysis of variance (ANOVA) using SPSS with significances of P <0.05, respectively.
Results
Salicylic acid inhibited hepatoma cell line proliferation
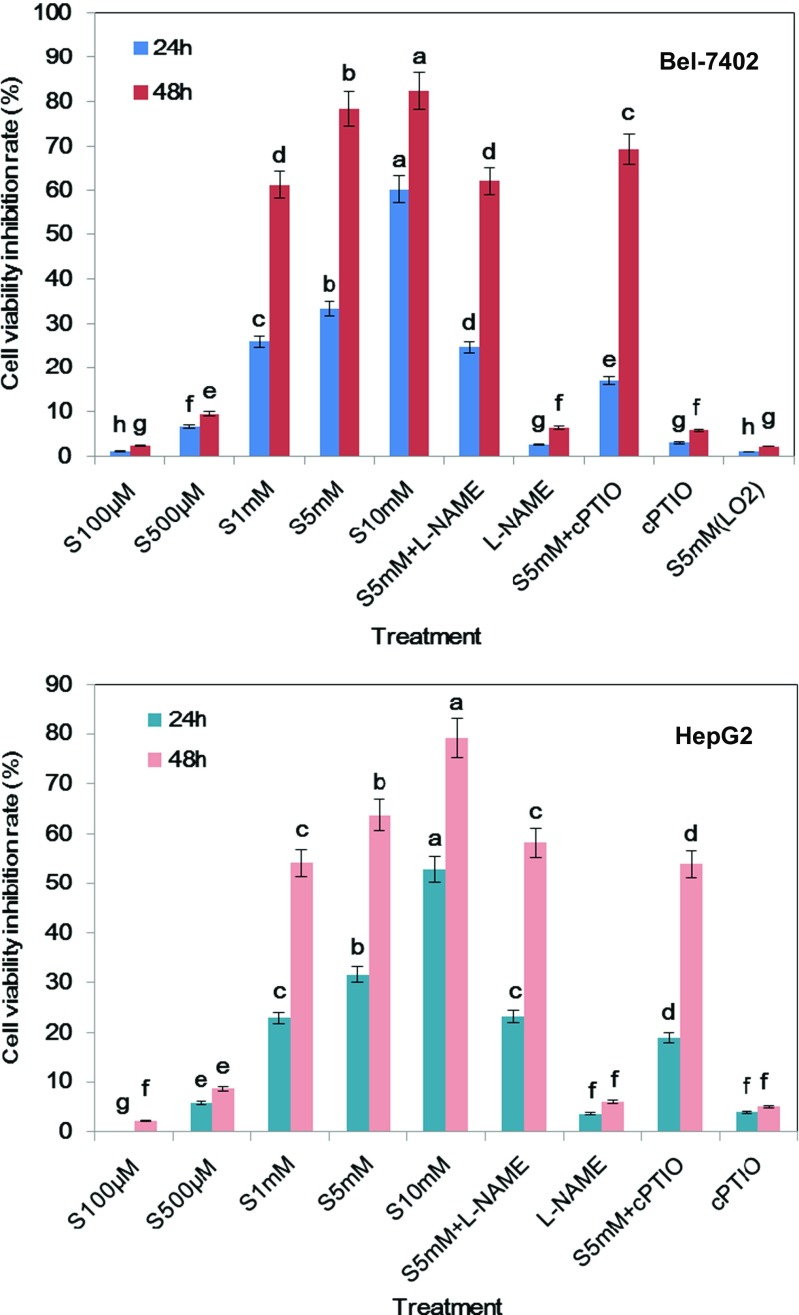
The MTS assay showed that the cell proliferation of Bel-7402 and HepG2 cancer cells was inhibited by SA treatment in a time- and dose-dependent manner (Fig. 1). Compared with the control group, SA at lower concentrations (100 and 500 μM) showed little inhibition effects on cell viability. However, the proliferation levels of 1, 5, and 10 mM SA treatment groups were obviously decreased at 24 and 48 h. In both cell lines, SA at the concentration of 5 mM caused less than half of cell growth inhibition at 24 h, but had little impact on the normal liver cell line LO2 (P > 0.05). Therefore, this concentration was used in all further experiments.
Fig. 1.
Inhibiting effects of SA on hepatoma cell line Bel-7402 and HepG2 growth after exposure of 24 and 48 h. SA, L-NAME (NOS inhibitor), and cPTIO (NO scavenger) were added to each well of a 96-well plate to yield the final concentrations. The cell viability inhibition rate of LO2 cell line with 5 mM SA treatment was also investigated. Cell viability was determined by the MTS assay and the absorbance was measured at 490 and 630 nm using a microplate reader. Results were expressed as percentages of proliferation compared to the control (mean ± SE, n = 6). Bars denoted by the different letters significantly differed at P <0.05 according to Duncan’s multiple range tests
Salicylic acid induced hepatoma cell line apoptosis
To investigate the anti-tumor effects of salicylic acid, the induction of apoptotic cell death was measured by Annexin V-FITC/PI assay after 24 h of treatment with different concentrations of SA (Fig. 2). The analysis of the Bel-7402 cells demonstrated that there was an increase in the number of cells in the early stages of apoptosis (control, 2.5%; 100 μM, 3.7%; 500 μM, 6.8%, 1 mM, 8.5%; 5 mM, 12.7%; 10 mM, 14.5%). Moreover, treatments with higher concentrations of SA caused an obvious increase in the late stages of cell apoptosis (control, 3.8%; 5 mM, 25.8%; 10 mM, 32.6%). The apoptosis of HepG2 cells were also induced by SA at the 24 h time point (figure not shown).
Salicylic acid induced apoptosis in association with the release of NO
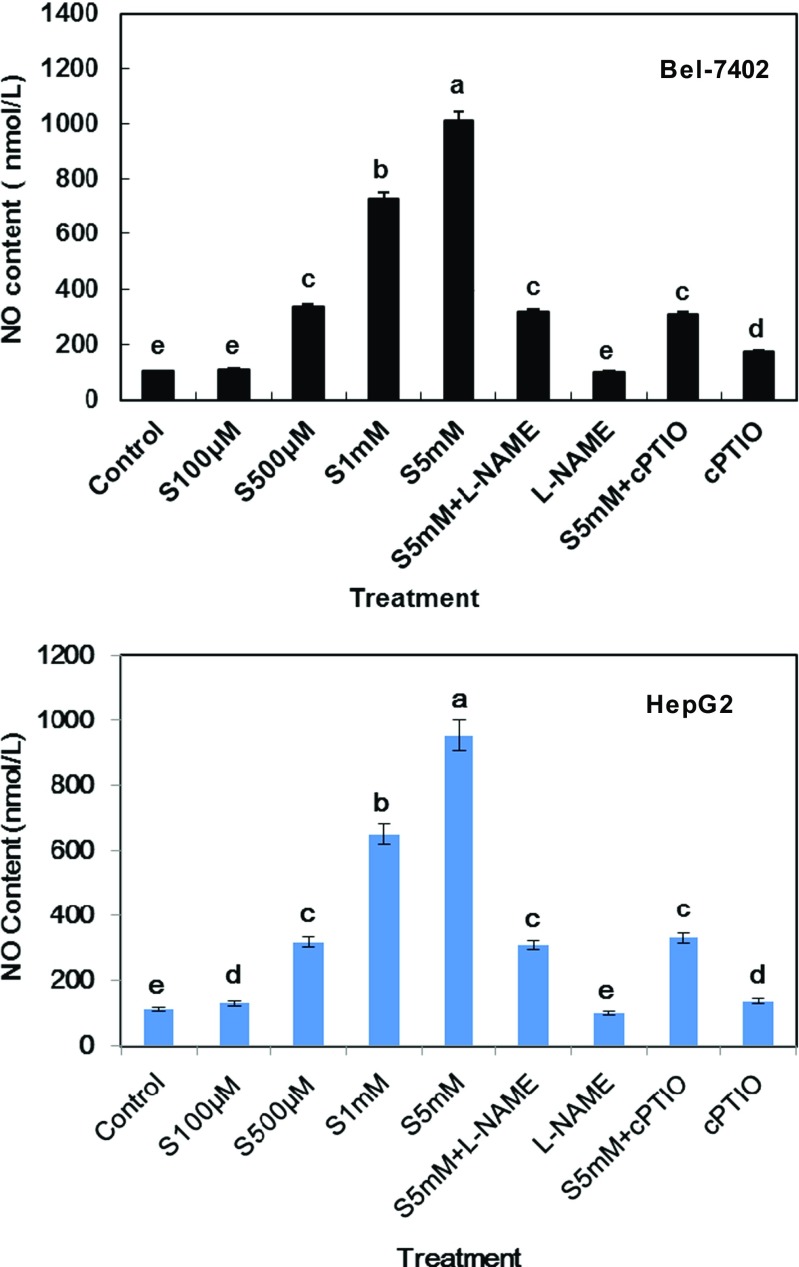
Nitric oxide (NO), a pleiotropic regulator, may have both genotoxic and angiogenic properties in tumor cells. In order to explore the role of NO in SA-induced apoptosis of hepatoma cell line, we assessed the effect of SA stimulation on NO production and discovered that SA had a dose-dependent inducing function on NO release (Fig. 3). For example, in the 100 μM, 500 μM, and 1 mM SA treatments for 24 h, the total NO population of each group were 109.8, 337.2, and 730.4 nmol/l, respectively in Bel-7402 cell line. With the increase in incubation concentration, 5 mM SA could lead to the maximum release of NO, approximately 6.7 times compared with control treatment. As predicted, increased NO production was also observed in HepG2 cell line in a concentration-dependent manner.
Fig. 3.
NO release in SA-induced apoptosis of Bel-7402 and HepG2 cell line after different treatments for 24 h. Bars denoted by the same letter did not significantly differ at P <0.05 according to Duncan’s multiple range tests
Salicylic acid induces the boost of NO in hepatoma cell line apoptosis related to the augment of NOS activities
In previous studies, we identified that SA increases NO production in hepatoma cells, but the underlying mechanism was not clear. Various studies have shown that nitric oxide synthases (NOS) stimulates angiogenesis by generating nitric oxide. In our study, we investigated the possibility that SA stimulates NOS expression and discovered that the extent of total NOS and inducible NOS activities were increment in both Bel-7402 and HepG2 cells, which were in accordance with NO content after different concentrations of SA treatments. For instance, in Bel-7402 cell line, 500 μM and 1 mM SA treatments for 12 h could bring about the slight increase of total NOS and inducible NOS activities (Fig. 4a, b). Moreover, the inducing effect of 5 mM SA treatment was the most significant, approximately up to 7.8 times compared with control. Besides, the level of NOS2 transcripts was also obviously higher in 5 mM SA treatment group (Fig. 4c).
Fig. 4.
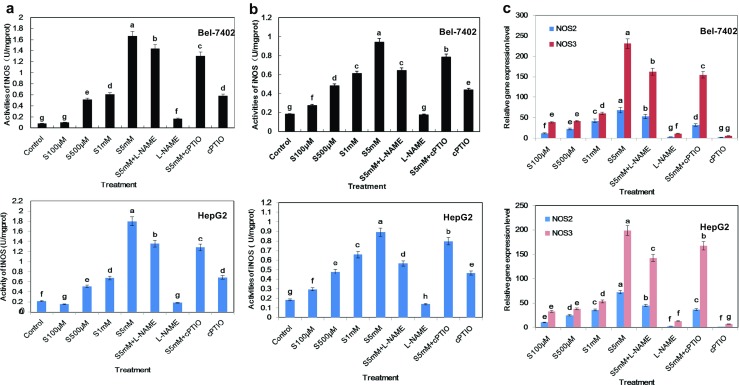
SA anti-tumor effects might mediate by NO pathway. Nitric oxide synthesis activities (a) and inducible nitric oxide synthesis activities (b) were determined by DAF-FMDA method after indicated treatments for 12 h. c Relative mRNA expression level of iNOS (NOS2) and eNOS (NOS3) in Bel-7402 and HepG2 cell line received indicated treatments above for 6 h. Bars denoted by the different letters significantly differed at P <0.05 according to Tukey’s multiple range tests
Salicylic acid up-regulated the expression of apoptotic regulator genes
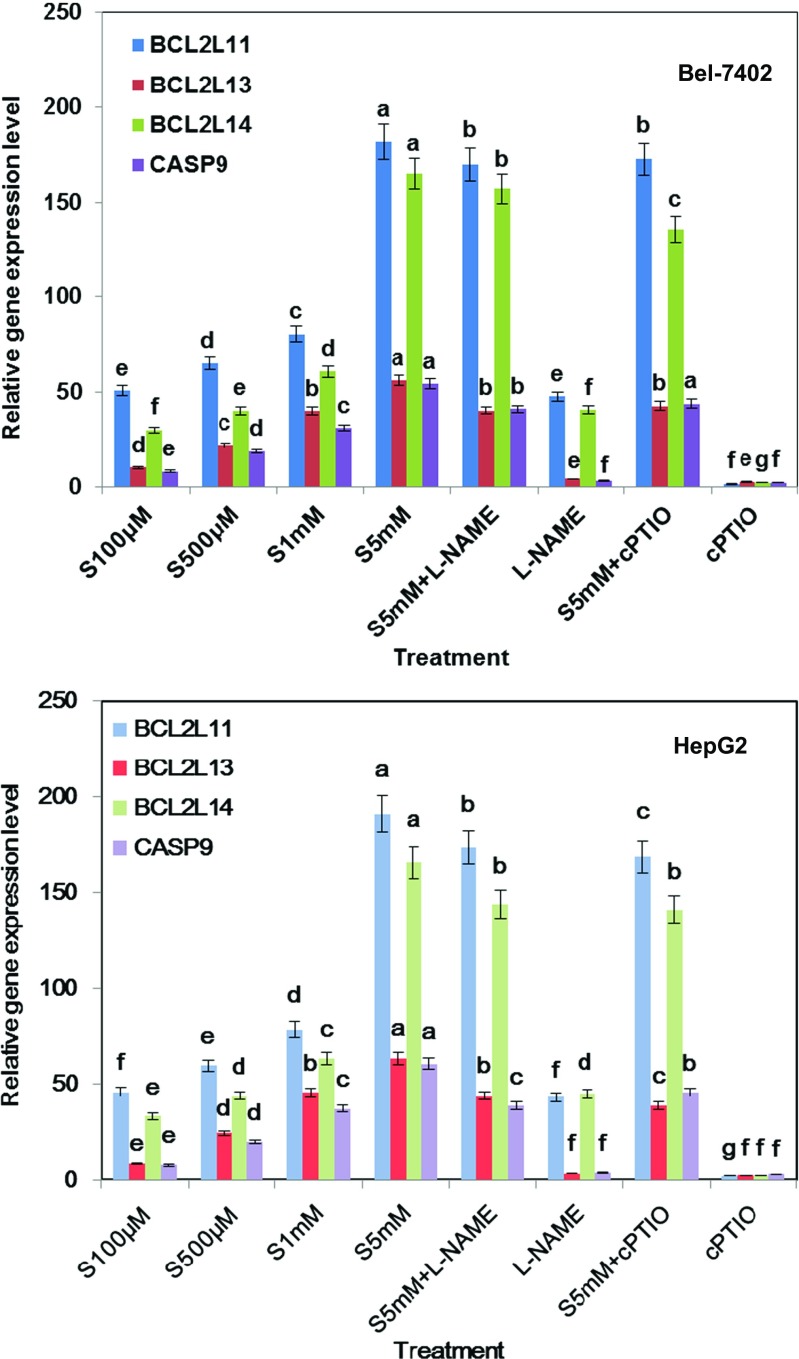
BCL-2 family members act as anti- or pro-apoptotic regulators which are involved in a wide variety of cellular activities. BCL-2L11, BCL-2L13, and BCL-2L14 have been shown to act as an apoptotic activator (Zamzami et al. 1998; Cory and Adams 2002; Misaka et al. 2015). To further explore the molecular mechanism involved in the significant anti-tumor effects of salicylic acid, Bel-7402 and HepG2 cell lines were collected and the change of apoptotic target genes with different concentrations of SA treatments was investigated. Real-time PCR analysis reveals that after being treated with 5 mM salicylic acid for 6 h, BCL-2L11, BCL-2L13, BCL-2L14, and CASP9 were obviously up-regulated (Fig. 5). However, lower concentrations of SA (100 μM, 500 μM) have slight inducing effects on these genes’ expression described above.
Fig. 5.
Apoptosis-related gene expression levels were up-regulated after SA treatment for 12 h in Bel-7402 and HepG2 cell line, respectively. The mRNA expression levels of BCL-2-Like11, 13, 14, and Caspase 9 in the indicated cells were measured by real-time PCR compared with control treatment. Bars denoted by the different letters significantly differed at P <0.05 according to Tukey’s multiple range tests
L-NAME, the NOS inhibitor, and cPTIO, NO scavenger, relieved SA-induced changes in the apoptosis of hepatoma cell line
Our previous study validated that NO level and NOS expression were elevated in SA-induced hepatoma cell line apoptosis. Further, L-NAME and cPTIO were used to evaluate whether NO modulated SA-induced hepatoma cell apoptosis. Our experiment data suggested that L-NAME and cPTIO administration significantly diminished not only the proliferation inhibition (Figs. 1 and 2) effects but also relative gene mRNA levels induced by SA (Figs. 5 and 6). L-NAME and cPTIO treatment alone showed little function.
Fig. 2.
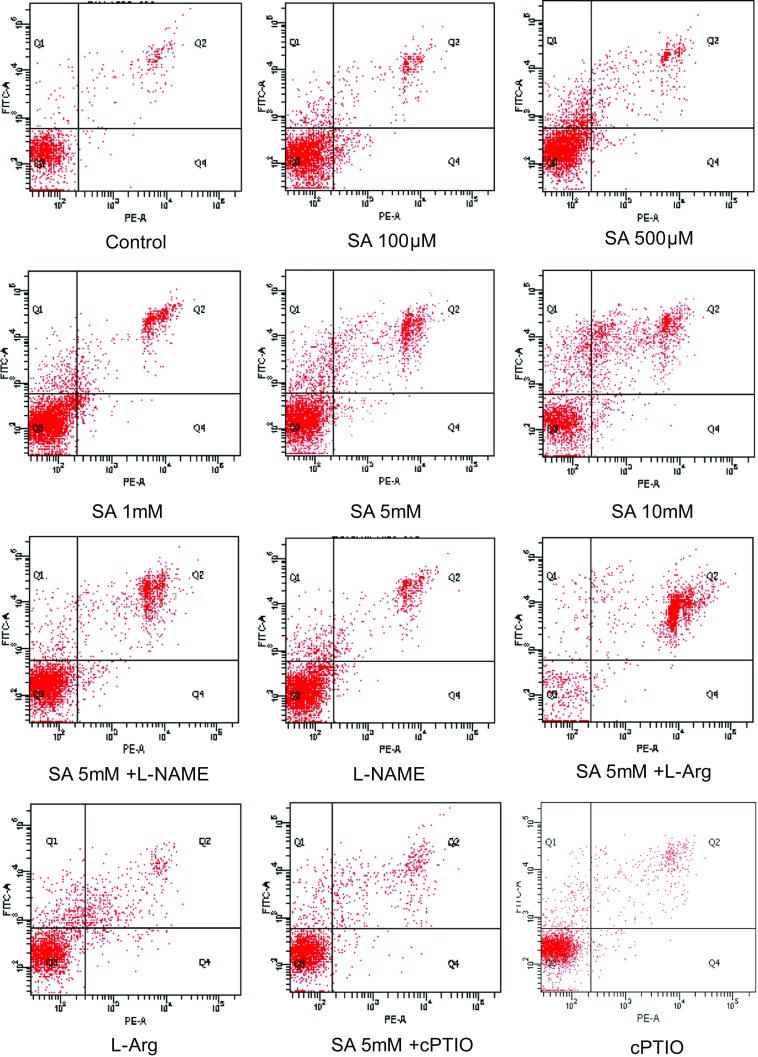
Cell apoptosis of Bel-7402 with different treatments for 24 h were determined by flow cytometry and dyed by Annexin V/PI double staining
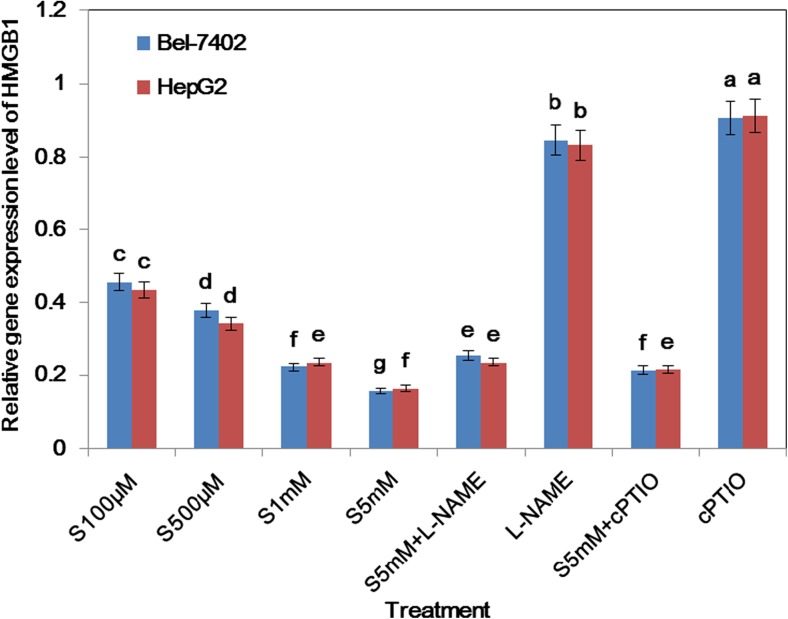
Fig. 6.
The transcripts levels of HMGB1 were depressed with different treatments for 6 h in Bel-7402 and HepG2 cell line, respectively. Bars denoted by the different letters significantly differed at P <0.05 according to Tukey’s multiple range tests
Salicylic acid depresses the transcript levels of HMGB1
In order to investigate the mechanisms as to how SA induces apoptotic regulator gene expression in hepatoma cell line apoptosis, the transcript level of HMGB1 was determined. As shown in Fig. 6, the expression of HMGB1 was decreased at 6 h exposed to different doses of SA treatment. Additionally, L-NAME and cPTIO combination reverses the depression effect of HMGB1 exerted by SA.
Discussion
Accumulating lines of evidence showed that salicylic acid, one of the aspirin metabolites, could inhibit the occurrence and development of cancer (Chung et al. 2003; Elder 1996; Klampfer 1999; Zitta et al. 2012). However, the related mechanisms about anti-liver carcinoma effects of salicylic acid were rarely conducted. Our research demonstrated that SA could inhibit the proliferation and induce the apoptosis of hepatoma cell line in a time- and dose-dependent manner, which implies a potential application of SA as an anti-cancer agent.
Nitric oxide (NO) in cancer cells was produced at sub-micromolar levels to activate signaling pathways leading to evasion of apoptosis on the one hand and promotion of cell proliferation, migration, and metastatic invasion on the other (Tan et al. 2013; Li et al. 2015). A few reports (Karmohapatra et al. 2007) have demonstrated that aspirin could activate nitric oxide synthase from human blood platelets. In this study, we also provided the evidence that SA could induce NO release generated by NOS in the apoptosis process of hepatoma cell line. In addition, the ameliorating effects of SA were prevented when NOS inhibitor L-NAME and NO scavenger cPTIO were administrated. These results might propose a link between NO generation and cell viability inhibition due to SA treatment.
Furthermore, we gained insight into the molecular mechanism of the cytotoxic effect exerted by SA on hepatoma cell line. A series of genes as stimulators which will trigger the apoptotic process might participate in this pathway. BCL-2-Like11, 13, and 14 are key members of apoptosis-related genes in mitochondrial apoptosis pathway, which are able to promote apoptosis. Therefore, the expressions of BCL-2-Like11, 13, and 14 were selected as apoptosis markers for anti-tumor medicine examination in many studies (Zamzami et al. 1998; Cory and Adams 2002; Misaka et al. 2015). By using HCC cell lines, we provided the evidence that SA increased the mRNA expressions of BCL-2-Like11, 13, and 14 while activating the caspase-9 cascade, which in turn leads to toxic effects on inducing liver cancer cell apoptosis.
Inside cells, HMGB1 is found mainly in the cell nucleus, where it participates in replication, recombination, transcription, and DNA repair processes. Following release into the extracellular space, HMGB1 becomes a proinflammatory cytokine which stimulates formation of new blood microvessels, enhances cell migration, activates the inflammatory condition, and affects cell proliferation (Jiang and David 2006). In our study, we discovered that SA could suppress the expression of HMGB1 from hepatoma cells and inhibiting NOS will increase HMGB1 mRNA level, which suggests that HMGB1 may act as a downstream effector of NO.
Based on these results, we deduced that SA triggered the NO pathway by activation of NOS expression and depression of HMGB1 mRNA level, which in the end led to induce the apoptosis of hepatoma cell line.
Conclusion
In summary, our findings highlighted a novel mechanism for SA to anti-liver carcinoma and for the first time suggested that the apoptosis process of hepatoma cell line by SA is partly mediated via NO pathway, which up-regulated the apoptosis corresponding gene expression. Therefore, it could be a potential therapeutic agent for HCC treatment in the future.
Acknowledgements
This work was supported by Ningbo Medical Project Foundation (No. 2011B05) and the Major Science and Technology Planning Program of Ningbo (No. 2012C50013).
Abbreviations
- SA
Salicylic acid
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- L-NAME
NG-nitro-l-arginine methyl ester hydrochloride
- cPTIO
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt
Contributor Information
Yahui Liu, Phone: +86-0574-87085074, Email: 702820686@qq.com.
Yong Wang, Phone: +86-0574-87085074, Email: 271825234@qq.com.
Li Li, Phone: +86-0574-87085074, Email: Lilyningbo@163.com.
Reference
- Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- Annemijn MA. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Choi HW, Tian M, Song F, Venereau E, Preti A, Park SW, Hamilton K, Swapna GV, Manohar M, Moreau M, Agresti A, Gorzanelli A, De Marchis F, Wang H, Antonyak M, Micikas RJ, Gentile DR, Cerione RA, Schroeder FC, Montelione GT, Bianchi ME, Klessig DF (2015) Aspirin’s active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses. Mol Med 18(21):526–535. doi:10.2119/molmed.2015.00148 [DOI] [PMC free article] [PubMed]
- Chung YM, Bae YS, Lee SY (2003) Molecular ordering of ROS production, mitochondrial changes, and caspase activation during sodium salicylate-induced apoptosis. Free Radic Biol Med 15;34(4):434-42. [DOI] [PubMed]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Elder DJE. Differential growth inhibition by the aspirin metabolite salicylate in human colorectal tumor cell lines: enhanced apoptosis in carcinoma and in vitro-transformed adenoma relative to adenoma relative to adenoma cell lines. Cancer Res. 1996;56:2273–2276. [PubMed] [Google Scholar]
- Elwood PC. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M, Dolwani S. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016 doi: 10.1371/journal.pone.0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci P, Fried M, Labrecque D. World gastroenterology organisation guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis. 2010;19:311–317. [PubMed] [Google Scholar]
- Giampieri R, Restivo A, Pusceddu V, Del Prete M, Maccaroni E, Bittoni A, Faloppi L, Andrikou K, Bianconi M, Cabras F, Berardi R, Zorcolo L, Scintu F, Cascinu S, Scartozzi M. The role of aspirin as antitumoral agent for heavily pretreated patients with metastatic colorectal cancer receiving capecitabine monotherapy. Clin Colorectal Cancer. 2016 doi: 10.1016/j.clcc.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Hagos GK, Carroll RE, Kouznetsova T, Li Q, Toader V, Fernandez PA, Swanson SM, Thatcher GR. Colon cancer chemoprevention by a novel NO chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6(8):2230–9. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92:4392–6. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WW, David S. The role of IFN-a and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol. 2006;177:3337–3343. doi: 10.4049/jimmunol.177.5.3337. [DOI] [PubMed] [Google Scholar]
- Karmohapatra SK, Chakraborty K, Kahn NN, Sinha AK. The role of nitric oxide in aspirin induced thrombolysis in vitro and the purification of aspirin activated nitric oxide synthase from human blood platelets. Am J Hematol. 2007;82(11):986–95. doi: 10.1002/ajh.20955. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16(19):2373–94. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- Klampfer L. Sodium salicylate activates caspases and induces apoptosis of myeloid leukemia cell lines. Blood. 1999;93:2386–2394. [PubMed] [Google Scholar]
- Klessig DF. Newly identified targets of aspirin and its primary metabolite, salicylic acid. DNA Cell Biol. 2016;35(4):163–6. doi: 10.1089/dna.2016.3260. [DOI] [PubMed] [Google Scholar]
- Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–903. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1897::AID-CNCR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Li W, Han W, Ma Y, Cui L, Tian Y, Zhou Z, Wang H. P53-dependent miRNAs mediate nitric oxide-induced apoptosis in colonic carcinogenesis. Free Radic Biol Med. 2015;85:105–13. doi: 10.1016/j.freeradbiomed.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Mc Menamin ÚC, Cardwell CR, Hughes CM, Murray LM. Low-dose aspirin and survival from lung cancer: a population-based cohort study. BMC Cancer. 2015;15:911. doi: 10.1186/s12885-015-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;15:2417–23. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Paterson JR, Lawrence JR. Salicylic acid: a link between aspirin, diet and the prevention of colorectal cancer. QJM. 2001;94:445–448. doi: 10.1093/qjmed/94.8.445. [DOI] [PubMed] [Google Scholar]
- Puglisi MA, Cenciarelli C, Tesori V, Cappellari M, Martini M, Di Francesco AM, Giorda E, Carsetti R, Ricci-Vitiani L, Gasbarrini A. High nitric oxide production, secondary to inducible nitric oxide synthase expression, is essential for regulation of the tumour-initiating properties of colon cancer stem cells. J Pathol. 2015;236(4):479–90. doi: 10.1002/path.4545. [DOI] [PubMed] [Google Scholar]
- Reveneau S, Arnould L, Jolimoy G, Hilpert S, Lejeune P, Saint-Giorgio V, Belichard C, Jeannin JF. Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Lab Invest. 1999;79:1215–25. [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Tan J, Zeng Q, Jiang XZ, He LY, Wang JR, Yao K, Wang CH. Apoptosis of bladder transitional cell carcinoma T24 cells induced by adenovirus-mediated inducible nitric oxide synthase gene transfection. Chin J Cancer Res. 2013;25(5):593–9. doi: 10.3978/j.issn.1000-9604.2013.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkala M, Kahlos K, Lakari E, Paakko P, Kinnula V, Soini Y. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in situ and invasive breast carcinomas. Clin Cancer Res. 2000;62:408–16. [PubMed] [Google Scholar]
- Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, DeGraff W, Gamson J, Vodovotz Y, Russo A, Mitchell JB. Nitric oxide and some NO donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1(1):88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu L. Nitric oxide: from a mysterious labile factor to the molecule of the Nobel Prize. Recent progress in nitric oxide research. Cell Res. 1998;8:251–8. doi: 10.1038/cr.1998.25. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16(17):2265–82. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- Zitta K, Meybohm P, Bein B, Huang Y, Heinrich C, Scholz J, Steinfath M, Albrecht M. Salicylic acid induces apoptosis in colon carcinoma cells grown in-vitro: influence of oxygen and salicylic acid concentration. Exp Cell Res. 2012;318(7):828–834. doi: 10.1016/j.yexcr.2012.02.002. [DOI] [PubMed] [Google Scholar]