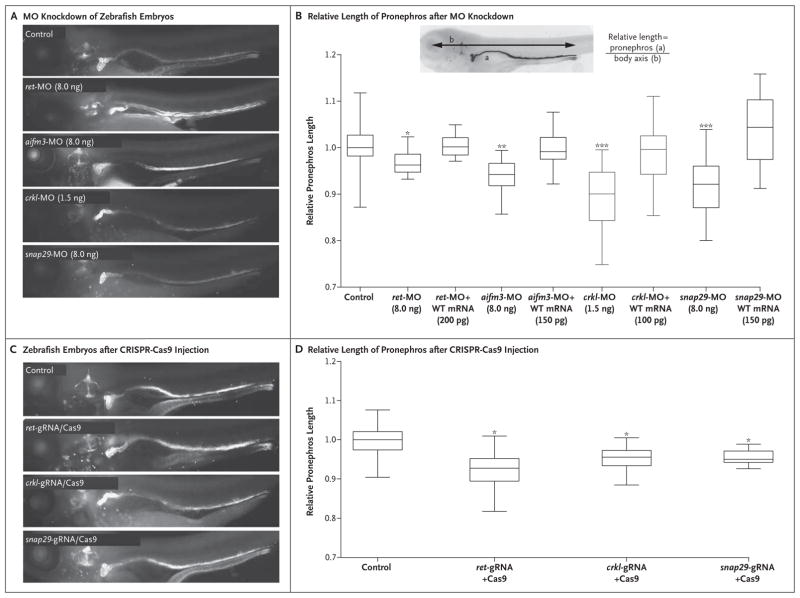
Figure 2. Functional Modeling of the DiGeorge Syndrome Terminal Deletion Genes Associated with Kidney and Urinary Tract Malformations.
Panel A shows zebrafish larvae 4.5 days after fertilization, in which the proximal tubule is folded into a hairpin structure, displaying proper anterior convolution in noninjected control embryos (staining with antibody against sodium–potassium ATPase). Knockdown of ret, aifm3, crkl, and snap29 by the injection of 8.0 ng of a splice-blocking morpholino oligonucleotide (MO) against RET resulted in major convolution defects, which are apparent by the failure of the anterior portion of the pronephros (the earliest developmental stage in the zebrafish) to progress, along with an overall reduction in the length of the tubules. Panel B shows the relative length of the pronephros, which was defined as the ratio of the length of the pronephros (a) to the length of the body axis (b), in individual larvae (inset). The number of replicate measurements were as follows: control or sham-injected control, 177 in Panel A and 68 in Panel B; ret-MO, 50; ret-MO+mRNA, 42; aifm3-MO, 38; aifm3-MO+mRNA, 42; crkl-MO, 43; crkl-MO+mRNA, 58; snap29-MO, 48; snap29-MO+mRNA, 39; ret-gRNA+Cas9, 44; crkl-gRNA+Cas9, 31; and snap29-gRNA+Cas9, 41). Morphant phenotypes could be rescued by the coinjection of each respective human messenger RNA (mRNA). In each box-and-whisker plot, the horizontal line represents the median, the top and bottom of the boxes the interquartile range, and the I bars the minimum and maximum values. Panel C shows embryos that have been injected with CRISPR–Cas9 and that are reproducing the convolution defects observed in the morphant embryos. Guide RNA (gRNA) that targeted each respective gene was coinjected with purified Cas9 protein, and the relative length of the pronephros was measured in founders, as shown in Panel D. In Panels B and D, a single asterisk indicates P<0.05, two asterisks P<0.01, and three asterisks P<0.001. WT denotes wild type.