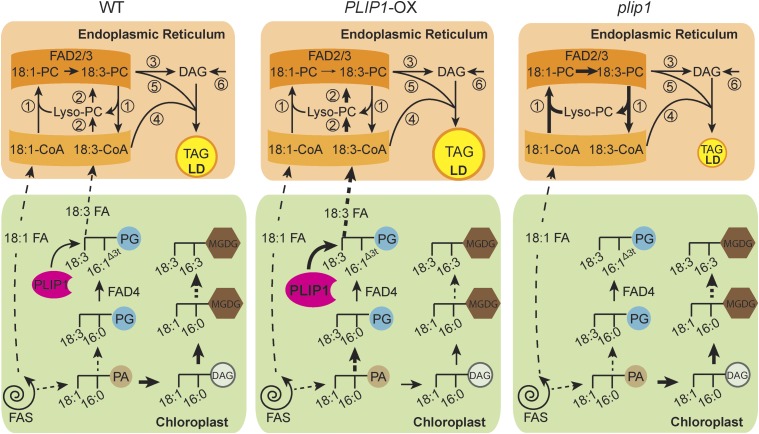
Figure 10.
Hypothesis for the Role of PLIP1 in Triacylglycerol Biosynthesis.
The left panel depicts the wild type, the middle panel the PLIP1 overexpression lines, and the right panel the plip1 mutant. The thickness of the arrows indicates the relative fluxes in the three different lines. Reactions or sets of reactions are numbered as follows: (1) In the wild type (left panel), acyl exchange on PC involving desaturation of acyl groups by FAD2/3 provides one mechanism to introduce polyunsaturated FAs into PC. (2) A parallel mechanism to introduce PUFAs into PC involves PLIP1. In the chloroplast, PLIP1 hydrolyzes 18:3/16:1Δ3t- PG and other 16:1Δ3t-phosphatidylglycerol species at the sn-1 glyceryl position and releases 18:3 (carbon:double bonds) or other acyl groups at the sn-1 position. The released acyl group is exported to the ER and incorporated into the acyl-CoA pool and PC. (3) A head group exchange mechanism leads to diacylglycerol (DAG) formation from PC containing PUFAs. (4) TAG, which accumulates in lipid droplets (LDs), is formed by the action of DAG-acyltransferases, which can introduce additional acyl groups into DAG from the acyl-CoA pool. (5) Phospholipid-DAG acyltransferase provides an additional route for the incorporation of polyunsaturated FAs from PC into TAG. (6) DAG can also be formed by de novo assembly through the Kennedy pathway, which, however, is thought to play a minor role in the synthesis of TAGs in seeds. In the chloroplast, biosynthesis of PG and MGDG shares the precursor phosphatidic acid (PA), with more PA being shuttled to MGDG biosynthesis in the wild type. In PLIP1-OX lines (middle panel), both PG biosynthesis and degradation are accelerated, resulting in increased export of 18:3 and other acyl groups and their direct incorporation into PC (reaction 2). Direct incorporation of 18:3 competes with polyunsaturated FA formation by the acyl-editing pathway of PC involving FAD2/3 (reaction 1), but leads to increased flux of 18:3 into the end product TAG. As a result of increased PG turnover in chloroplasts of PLIP1-OX lines, PA is preferably shuttled into PG biosynthesis, which subsequently reduces its availability for MGDG assembly in the plastid visible in changes in the MGDG acyl composition. In the plip1 mutant (right panel), the PLIP1-dependent pathway is deficient, resulting in decreased TAG biosynthesis. Without the competing effect of PLIP1 on the acyl exchange reactions and FAD2/3, more 18:1 is converted to 18:3, explaining the altered acyl composition of TAG and other extraplastidic lipids.