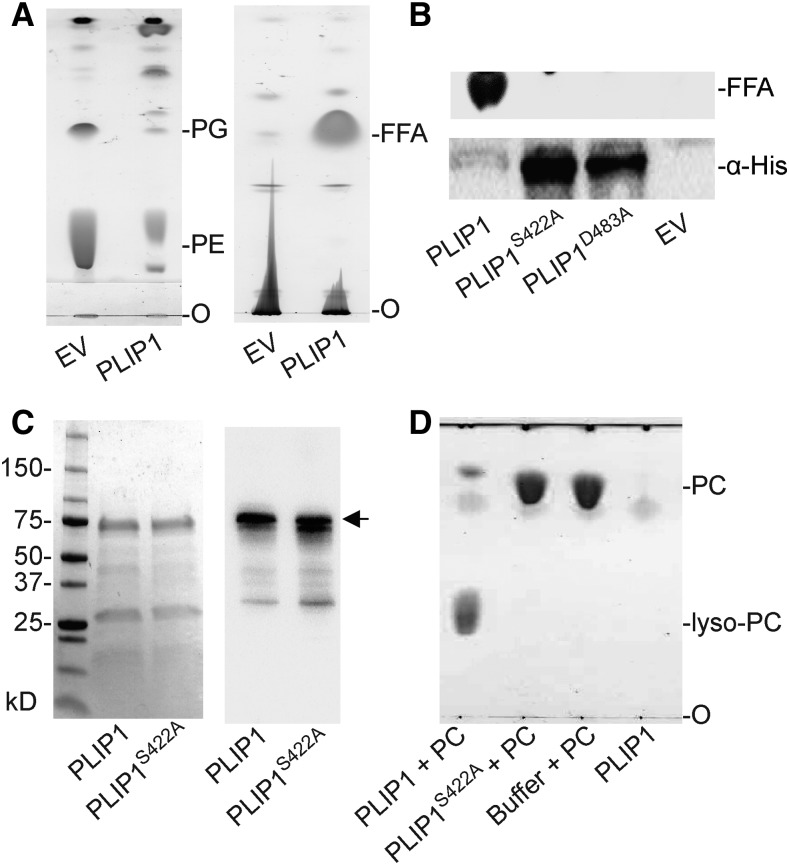
Figure 2.
Recombinant PLIP1 Has Lipase Activity.
(A) Thin-layer chromatographic analysis of polar (left) and neutral (right) lipids in E. coli containing a 6×His-PLIP1 expression construct or EV control at 6 h following induction. FFA, free fatty acid; O, origin of sample loading. TLC plates were stained with iodine vapor.
(B) Mutation of the PLIP1 active site motif. Lipid extracts of E. coli cultures 6 h after induction expressing 6×His-PLIP1 or two point mutation alleles, 6×His -PLIP1S422A or 6×His-PLIP1D483A, or containing an EV control were analyzed by TLC to detect FFA products (top panel). Protein extracts were analyzed for protein production using an antibody against the 6×His tag.
(C) SDS-PAGE analysis of purified PLIP1 and PLIP1S422A proteins. Loading was 5 µg per lane for both samples. SDS-PAGE separated proteins were stained by Coomassie blue (left) or detected by immunoblotting with an antibody raised against PLIP1S422A (right). Numbers indicate protein molecular mass in kilodaltons. 6×His-PLIP1 and 6×His-PLIP1S422A are indicated by the arrow.
(D) Thin-layer chromatogram of products of a representative in vitro lipase reaction using PC with the wild type (PLIP1 + PC) and the mutant enzyme (PLIP1S422A + PC). Substrate without enzyme (Buffer + PC) and enzyme without substrate (PLIP1) were included as controls. O, origin of sample loading. Lipids were visualized by iodine vapor staining.