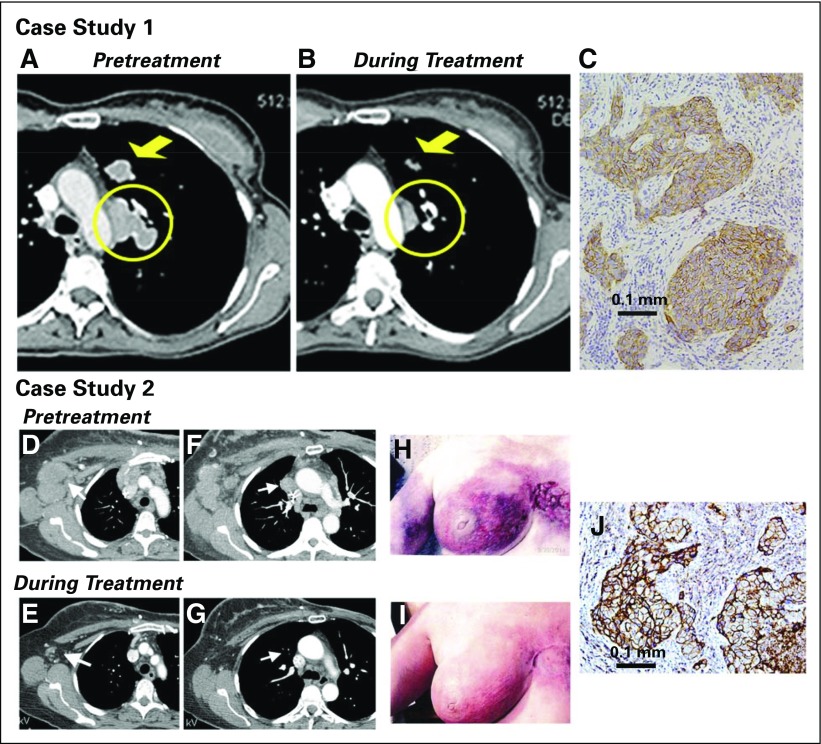
Fig 2.
Examples of patients with objective responses. Case study 1: A 48-year-old woman with an initial diagnosis of triple-negative breast cancer in November 2007 received four prior lines of treatment (two lines in a metastatic setting, including an anti-PD-L1 immune checkpoint inhibitor) and presented with lung and lymph node metastases at the time of enrollment in February 2015. She achieved a partial response that started 1.7 months after initiation of treatment with sacituzumab govitecan, with a best response of 54% reduction at 9.0 months and progression occurring at 14.4 months (partial response duration, 12.7 months). (A) Baseline image of two of the three target lesions: a 24 × 19-mm left-upper-lung mass (arrow) and a mediastinal lymph node (17 × 29-mm; circle). (B) After 16 doses (July 2015), these two target lesions decreased to 13 × 7 and 9 × 19 mm, respectively. (C) Trop-2 expression in an archived tumor specimen by immunohistochemistry that shows 1+ to 2+ staining (overall, 2+). Case study 2: A 67-year-old woman with an initial diagnosis of estrogen receptor–positive breast cancer in 2007 that was treated by lumpectomy and local radiation. In 2012, a local recurrence was treated with neoadjuvant doxorubicin and cyclophosphamide followed by taxol and a mastectomy. One year later, the patient received a diagnosis of metastatic disease, which was biopsy-proven triple-negative breast cancer. She was enrolled in a clinical trial and had stable disease for 3 months, but she discontinued treatment because of progression, including new brain metastases. After stereotactic radiosurgery and three cycles of eribulin, the patient progressed and was enrolled in the current study. Her brain metastases had been stable for > 3 months at the time of enrollment. (D) and (F) Baseline (September 2014) and (E) and (G) 7.2-month follow-up (April 2015) computed tomography scans performed after 22 treatments that showed two target lesions: (D) and (E) axillary and (F) and (G) mediastinal lymph nodes (arrows). Her first computed tomography response assessment in November 2014 showed a 41% reduction (partial response) after just six doses, which was confirmed 1 month later, and at this time, magnetic resonance imaging showed no evidence of intracranial metastases. (H) Photograph of the patient’s skin involvement at the onset of treatment (September 2014) and (I) a photograph that shows a response in December 2014. The patient continued treatment, with 66% reduction as the overall best response recorded in September 2015 and with four of the five nontarget lesions remaining stable and one completely resolved (supraclavicular lymph node). However, in October 2015, two of the nontarget lesions progressed, and she was withdrawn from the study. (J) Trop-2 expression in archived tumor by immunohistology that shows 3+ staining.