Fig. 2.
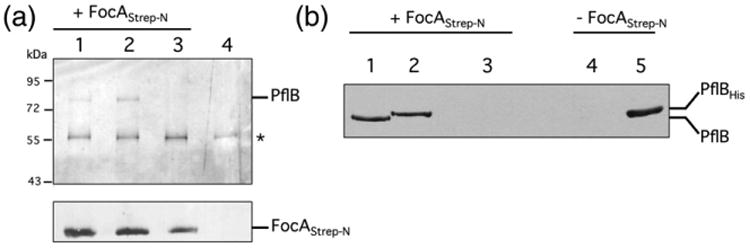
Protein–protein interaction studies with purified Strep-tagged FocA. (a) Purified N-terminally Strep-tagged FocA (10 μg of protein) was incubated with the soluble fraction (1 mg of protein) derived from strains MC4100 (lane 1), REK702 (lanes 2 and 4) or RM220 (ΔpflB) (lane 3), which had been grown anaerobically (see Materials and Methods). Equivalent amounts of suspension eluted from the Streptactin matrix were separated by 10% (w/v) (upper panel) or 12.5% (w/v) (lower panel) SDS-PAGE and challenged with antibodies directed against PflB or FocA, as indicated on the right side of each panel. Size markers in kDa are shown on the left (purified FocAStrep-N migrates with a molecular mass of 24 kDa) and the asterisk indicates the unidentified polypeptide that cross-reacts with the anti-PflB antibodies. (b) Purified Strep-tagged FocA (20 μg of protein) was incubated with 100 μg of soluble fraction derived from strains MC4100 containing plasmid p29 (resulting in overproduction of PflB) (lanes 1 and 4), RM201 (ΔfocA ΔpflB) (lane 3) or purified His-tagged PflB (10 μg) (lane 2), and after release of FocA and its interaction partners, the eluate was applied to a 10% (w/v) SDS-PAGE and after transfer to nitrocellulose the membrane was challenged with anti-PflB antibodies. The migration positions of PflB and His-tagged PflB are shown on the right of the panel. (See also Fig. S1.)
