Figure 1. Identification of a Functional Pds5-binding Motif in Human Wapl and Sororin.
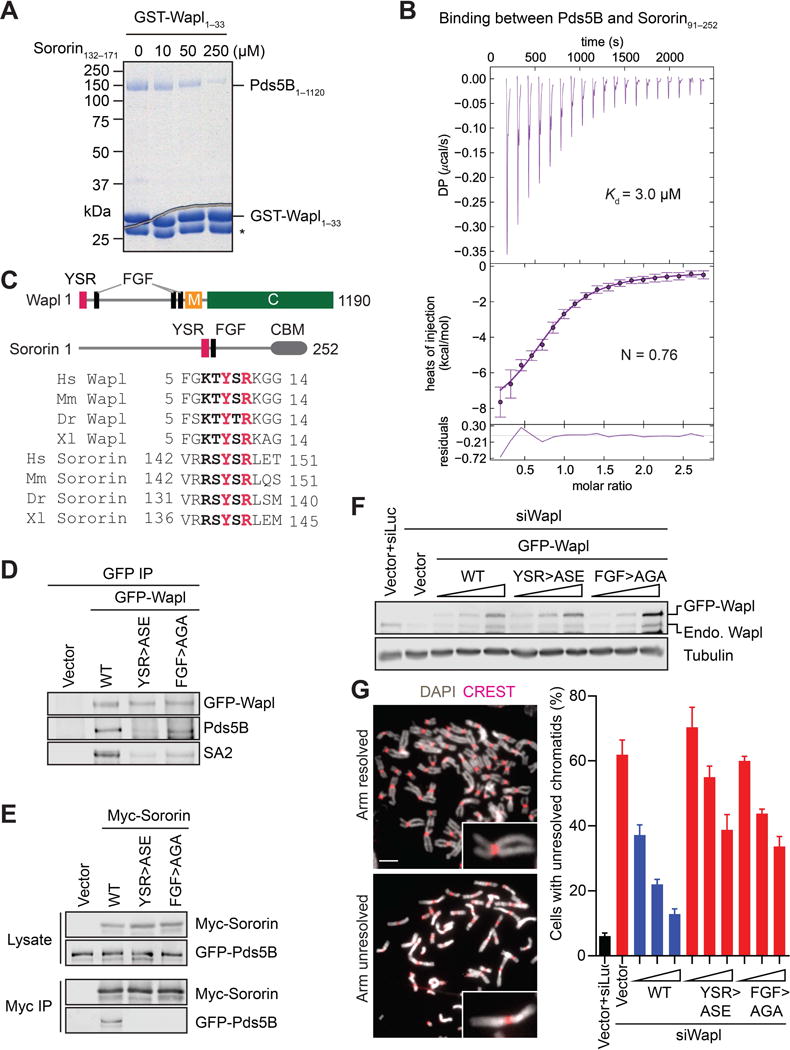
(A) Coomassie-stained SDS-PAGE gel of recombinant human Pds5B1–1120 bound to GST-Wapl1–33, in the absence or presence of increasing concentrations of a Sororin peptide. Asterisk indicates a proteolytic fragment of GST-Wapl1–33.
(B) Isothermal titration calorimetry (ITC) curves of the binding between purified recombinant human Pds5B1–1120 and Sororin91–252, with Kd and binding stoichiometry (N) indicated. DP, differential power.
(C) Schematic drawing of domains and motifs of human Wapl and Sororin, and sequence alignment of the YSR motifs of Wapl and Sororin from human (Hs), mouse (Mm), zebrafish (Dr), and Xenopus (Xl). CBM, cohesin-binding motif.
(D) Anti-GFP, anti-Pds5B, and anti-SA2 blots of anti-GFP immunoprecipitates (IP) of HeLa cells transfected with the indicated GFP-Wapl plasmids. WT, wild type; ASE, Y9A/R11E; AGA, F73A/F75A.
(E) Anti-Myc and anti-GFP blots of lysates and anti-Myc IP of HeLa cells transfected with plasmids encoding GFP-Pds5B and the indicated Myc-Sororin proteins. WT, wild type; ASE, Y146A/R148E; AGA, F166A/F168A.
(F) Anti-Wapl and anti-β-tubulin immunoblots of lysates of HeLa cells that were transfected with Wapl siRNA and increasing amounts of the indicated GFP-Wapl plasmids. Endo, endogenous. The increase of the untagged Wapl band intensity in GFP-Wapl samples was due to proteolysis of GFP-Wapl proteins or internal translation start of the transgene.
(G) Representative metaphase spreads of cells in (E) with arm-resolved or arm-unresolved chromosomes. Spreads were stained with DAPI (gray) and the kinetochore marker CREST (red). Selected sister chromatids are magnified in inset. Scale bar, 5 μm. Quantification of the percentages of mitotic cells in (E) that had arm-unresolved chromosomes. Error bars, SD (n = 3 independent experiments) (see also Figure S1).
