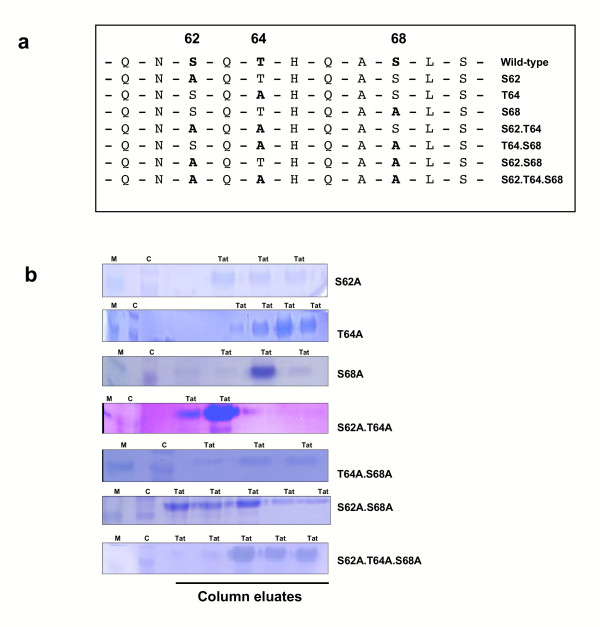
Figure 3.
Construction of HIV-1 Tat phosphorylation mutants. (a) Amino acid sequence of HIV-1 Tat wild-type and mutants. Changes to alanine at serine 62, threonine 64 and serine 68 are indicated for each mutant, and compared to the wild-type protein. Mutations were introduced by site-directed mutagenesis into pET-DEST42-HIS-Tat86. (b) Competent BL21(DE3)pLysS cells, transformed with pET-DEST42-HIS-Tat86 wild-type or mutants, were grown and lysed with 6 M guanidine-HCl, pH 8.0. The suspension was cleaned of cell debris and loaded onto a packed metal affinity resin. The resin was washed and the HIS-tagged Tat proteins were eluted with 6 M guanidine-HCl, pH 4.0. The fractions collected were dialysed in 0.1 mM DTT and then analysed by 15% SDS-PAGE and stained with Coomassie blue. Tat lanes show fractions containing HIS-tagged Tat proteins; M lanes, 14 kDa marker; C lanes, BL21(DE3)pLysS cell extract.