Figure 4.
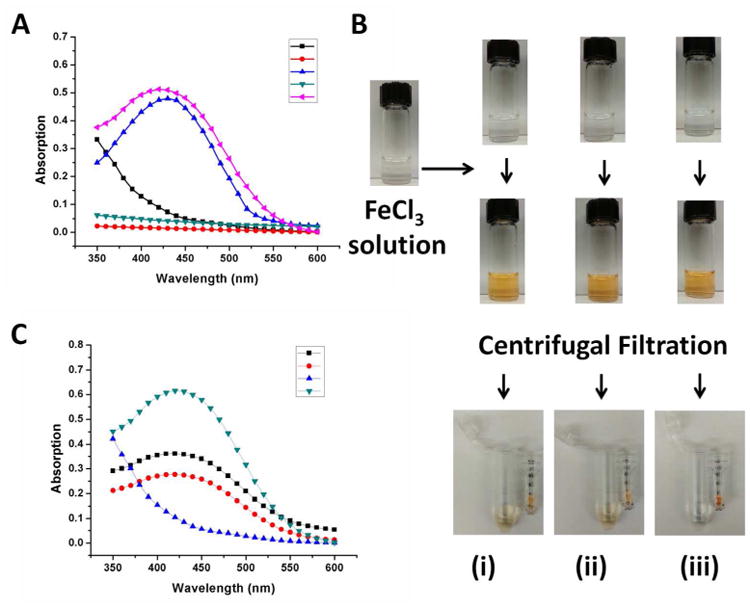
(A) UV-Vis absorption spectrum of Fe(III) in solution (black line), hCD-DFO in solution (red line), hCD-DFO in the presence of Fe(III) (blue line), hPR-DFO in solution (green line) and hPR-DFO in the presence of Fe(III) (pink line). (B) Optical images of DFO (i), hCD-DFO (ii) and hPR-DFO (iii) before addition of Fe(III) reveal clear solutions (first row of vials). After addition of iron, a distinct clear yellow-brown color forms immediately and is indicative of chelates (second row of vials). To further rule out the possibility that DFO was just loosely associated with PR, a microcentrifuge filter tube (MWCO 10,000 g/mol) was used to concentrate the material (third row of images); the yellow DFO/Fe(III) (i) or hCD-DFO/Fe(III) (ii) colored suspension passed through the filter into the bottom filtrate whereas the yellow colored suspension containing polymer chelates remained in the filter unit (iii). (C) UV-Vis absorption spectrum of the concentrate of hPR-DFO displays strong absorption at ca. 430 nm (green line) and no absorption for the filtrate (blue line) after extensive washing with the centrifugal filtration unit; the concentrates of DFO (black line) and hCD-DFO (red line) still absorb at ca. 430 nm but at lower magnitudes than before, reflecting their smaller sizes and elution through the membrane.
