Abstract
Background and aim
Caspase-cleaved cytokeratin 18 (CK18-Asp396) is a potential clinically useful biomarker in liver disease as it is released from hepatocytes during apoptosis. In this study, we investigated serum CK18-Asp396 levels in chronic hepatitis B (CHB).
Patients and methods
Overall, 163 patients with CHB were included. Serum CK18-Asp396 levels were determined by enzyme-linked immunosorbent assay (ELISA), and results were related to steatosis grade, histological activity index, inflammation score, and METAVIR fibrosis grade as well as to viral load, serum levels of liver enzymes, and albumin. Receiver operating characteristic analysis was used to evaluate the diagnostic performance of serum CK18-Asp396 levels for assessing disease activity.
Results
A higher level of serum CK 18 concentrations was found in patients with significant inflammation vs no significant inflammation (378.5 [interquartile range {IQR}: 173.2–629.6] vs 137.3 [87.5–197.7], P < 0.05; approximately threefold increase) and in patients with significant fibrosis vs no significant fibrosis (177.8 [IQR: 120.8–519.1] vs 142.7 [IQR: 88.8–214.4], P < 0.05; 1.25-fold increase). There was no differential CK 18 level by degree of steatosis. CK 18 was an independent predictor of significant inflammation with an 82% specificity and a 94% negative predictive value. We found the strongest correlation of CK 18 with alanine aminotransferase and aspartate aminotransferase (both r = 0.52; P < 0.001), but less with albumin (r = −0.24; P < 0.05) and viral load (log) (r = 0.19; P < 0.05).
Conclusion
CHB appears to be accompanied by continuous high levels of hepatocyte apoptosis as judged from serum CK 18, suggesting that elimination of the infected compartment constitutes a defensive strategy against disease. Accordingly, CK 18 works as an independent predictor of significant inflammation with a high specificity.
Keywords: CHB, CK 18, apoptosis, steatosis, inflammation
Plain language summary
In recent years, it has become clear that serum levels of a fragment of cytokeratin 18 (the so-called caspase-cleaved cytokeratin 18 [CK18-Asp396]) reflect the amount of programmed cell death in the liver. It remains, however, obscure whether programmed cell death is in general important during hepatitis B infection and specifically whether serum levels of CK18-Asp396 can be a marker for disease severity in hepatitis B. In this study, we showed that liver inflammation of hepatitis B is characterized by high levels of the so-called CK18-Asp396, and this novel marker for hepatitis B-associated inflammation is potentially superior to currently used serum markers.
Introduction
Chronic hepatitis B (CHB) infection is a major public health problem, which affects more than 350 million people worldwide.1 It is well known that CHB is associated with hepatic steatosis, inflammation, and fibrosis.2–5 Hence, histological examination is an integral part of evaluating CHB patients. Evaluation of the CHB-associated liver damage is critically important for making decisions in antiviral therapy and need for surveillance.6 Liver biopsy remains the golden standard in assessing the severity of inflammation and fibrosis.6,7 However, the procedure is costly, sensitive to sampling errors, and a potential cause for complications.8 Therefore, a reliable noninvasive diagnostic tool is urgently needed.
An increasing number of studies have emerged to explore potential noninvasive tools. Given the importance of apoptosis in the development of chronic liver disease in general,9–11 the importance of apoptosis in CHB remains unclear, and studies are hampered by the difficulty of distinguishing apoptosis in the hepatocyte compartment and the lymphocyte compartment. The former may represent the efforts of the body to limit the size of the infected compartment and may thus be associated with antiviral responses in lieu of viral clearance and consequently with aggravated inflammation and progression to hepatocellular carcinoma (HCC).12,13 Apoptosis in the lymphocyte compartment is, however, associated with reduced inflammatory activity.14 Hence, studies looking specifically at hepatocyte apoptosis are urgently needed to characterize the role of programmed cell death in CHB, and hepatocyte-specific apoptosis markers may also have substantial diagnostic value.
In this context, cytokeratin-18 (CK 18) is interesting. Expression of CK 18 is limited to certain endodermal derivatives, but is most prominently expressed in hepatocytes.15 During hepatocyte apoptosis, CK 18 is subject to specific cleavage by caspases, resulting in the release of neo-epitope (CK18-Asp396), which is not detectable in necrotic or vital cells.16 CK 18 is the best described hepatocyte-specific apoptosis marker,17 and many studies investigated the potential value of CK 18 as a noninvasive marker in predicting the severity of steatosis, inflammation, and fibrosis. Previous studies have shown that increased levels of CK18-Asp396 are associated with CHB and related to the severity of steatosis.18–20 In addition, CK18-Asp396 can be a predictive marker for distinguishing between inactive carrier and HBeAg-negative CHB,21 and CK 18 correlates with the presence of significant fibrosis in chronic hepatitis C,22 nonalcoholic fatty liver disease (NAFLD),23 and cirrhosis associated with CHB.24 Thus, CK 18 appears useful to measure hepatocyte apoptosis in CHB and its measurement would allow making correlations between hepatocyte programmed cell death, inflammatory responses, and viral load, allowing assessment as to the extent to which hepatocyte apoptosis contributes to antiviral defense.
In this study, we measured CK18-Asp396 in a group of CHB patients in a well-characterized Dutch cohort which includes patients across all grades of steatosis, inflammation, and fibrosis. The results show that CK18-Asp396 is associated with hepatic inflammation and diagnostically useful for clinical determination of this condition. Furthermore, the results support the notion that hepatocyte apoptosis may contribute to limiting the size of the virus-infected compartment.
Patients and methods
Patient selection
This retrospective study included 163 consecutive patients with CHB from 1985 to 2012 at the Erasmus University Medical Center in Rotterdam, the Netherlands. Three indications were defined as an end point in this study: steatosis grade, inflammation, and fibrosis. Steatosis was classified into the following groups according to Brunt score:25 <5%, normal; 5–33%, mild steatosis; 34–66%, moderate steatosis; >66%, severe steatosis. The inflammatory activity (grade) and the degree of hepatic fibrosis (stage) were assessed according to the modified histological activity index of Ishak system and METAVIR system (nil fibrosis, F0; mild fibrosis, F1; moderate fibrosis, F2; advanced fibrosis, F3; cirrhosis, F4), respectively.26,27 For the purpose of this study, inflammation was divided into two groups: significant inflammation (grade ≥ 7) and nonsignificant inflammation (grade < 7). Similarly, fibrosis staging was divided into subgroups: significant fibrosis (stage ≥ 3) and nonsignificant fibrosis (stage < 3).7
This study was performed according to the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice. Due to the retrospective nature of this study, the informed consent was not obtained from patients. Patients’ identities were not revealed in our study. This study was approved by the ethical review board of Erasmus Medical Center (Rotterdam, the Netherlands).28
Laboratory test
Serum level of CK 18 was measured by the M30-Apoptosense enzyme-linked immunosorbent assay (ELISA) kit (VLVbio [Peviva], Nacka, Sweden) according to the manufacturer’s instructions, which have been well established to accurately measure CK18-Asp396.29 Each patient sample was measured in triplicate, and the absorbance value was determined by microplate reader (FLUOstar Omega; BMG Labtech, De Meern, the Netherlands), according to the routine procedures.30 In short, the ELISA measures apoptosis in CK 18-positive cells, such as hepatocytes, with an antibody that specifically recognizes soluble caspase-cleaved CK 18.
Statistical analysis
Continuous variables were reported as mean (SD) or median (interquartile range [IQR]) according to data distribution, and categorical variables as percent. Quantitative variables were analyzed using Student’s t-test for normal distribution data or Mann–Whitney U test for highly skewed data. The eventual diagnostic predictor value was calculated by receiver operating curve (ROC). Youden index was used to determine the optimal cutoff value of CK 18. The probabilities of true positive (sensitivity) and true negative (specificity) were determined according to the calculated optimal cutoff value. The positive/negative likelihood ratio (LR+/LR−) was calculated by the following formula: LR+ = sensitivity/(1 – specificity); LR− = (1 – sensitivity)/specificity. The area under the ROC curve (AUROC) was generated to assess the diagnostic performance of each independent predictor. ROC relevant analyses were performed by using pROC package.31 Correlation was calculated using Spearman’s rank correlation coefficient. All statistical analyses were performed in R software (version 3.2.0), and statistical significance was set at P < 0.05 (two-tailed).
Results
Patient characteristics
The characteristics of the patients are described in Table 1. The mean age of the 163 patients was 40 years old. In terms of inflammation, there were 22 patients with significant inflammation (grade ≥ 7), which accounted for 13% of total patients. As for fibrosis, there were 33 cases with significant fibrosis (advanced fibrosis or cirrhosis), accounting for 20% of patients. Finally, the grade of steatosis was considered as normal in 2 patients (1%), mild in 104 patients (64%), moderate in 44 patients (27%), and severe in 13 patients (8%).
Table 1.
Clinical characteristics of patients with CHB virus infection
| Characteristics | All patients (n = 163) |
Significant inflammation (n = 22) |
No significant inflammation (n = 141) |
Significant fibrosis (n = 33) |
No significant fibrosis (n = 130) |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 136 (83) | 18 (18) | 118 (84) | 28 (85) | 108 (83) |
| Female | 27 (17) | 4 (82) | 23 (16) | 5 (15) | 22 (17) |
| Age, years | |||||
| Mean (SD) | 40.0 (11.5) | 45.5 (13.1) | 39.1 (11.0) | 47.2 (12.1) | 38.1 (10.6) |
| Median (IQR) | 41.0 (31.0–48.0) | 47.5 (35.3–55.8) | 39.0 (31.0–46.0) | 48.0 (39.0–56.0) | 37.0 (30.3–45.8) |
| Race, n (%) | |||||
| Caucasian | 84 (52) | 12 (55) | 72 (51) | 18 (54) | 66 (51) |
| Asian | 51 (31) | 8 (36) | 43 (30) | 11 (33) | 40 (31) |
| African/Black | 23 (14) | 1 (5) | 22 (16) | 4 (12) | 19 (15) |
| Other race | 5 (3) | 1 (5) | 4 (3) | 0 (0) | 5 (4) |
| BMI | |||||
| Mean (SD) | 27.2 (4.1) | 27.5 (4.0) | 27.2 (4.2) | 27.1 (3.3) | 27.3 (4.3) |
| Median (IQR) | 27.0 (24.8–29.8) | 28.6 (25.6–30.1) | 27.0 (24.8–29.8) | 27.2 (24.8–29.2) | 27.0 (24.8–29.8) |
| HBeAg status, n (%) | |||||
| Positive | 58 (36) | 13 (59) | 45 (32) | 11 (33) | 47 (36) |
| Negative | 105 (64) | 9 (41) | 96 (68) | 22 (67) | 83 (64) |
| ALT, xULN, U/L | |||||
| Mean (SD) | 2.3 (3.9) | 3.2 (1.8) | 2.2 (4.1) | 2.4 (1.8) | 2.3 (4.3) |
| Median (IQR) | 1.5 (1.1–2.2) | 2.8 (1.9–4.6) | 1.4 (1.1–1.9) | 1.8 (1.0–3.4) | 1.5 (1.1–2.0) |
| AST, xULN, U/L | |||||
| Mean (SD) | 1.4 (1.2) | 2.1 (0.9) | 1.2 (1.2) | 1.8 (1.1) | 1.3 (1.2) |
| Median (IQR) | 1.0 (0.8–1.4) | 1.9 (1.5–2.6) | 1.0 (0.8–1.2) | 1.5 (1.0 –2.1) | 1.0 (0.9–1.2) |
| ALK | |||||
| Mean (SD) | 79.9 (64.5) | 85.6 (33.8) | 78.9 (68.6) | 82.4 (28.5) | 79.2 (71.2) |
| Median (IQR) | 72 (60.0–89.0) | 72.0 (63.0–97.0) | 71.0 (59.0–86.0) | 75.0 (62.8–90.5 | 71.0 (59.0–84.0) |
| Albumin, g/L | |||||
| Mean (SD) | 45.0 (3.5) | 42.4 (3.1) | 45.5 (3.4) | 42.8 (4.0) | 45.7 (3.1) |
| Median (IQR) | 45.0 (43.0–47.0) | 42.5 (40.0–45.0) | 46.0 (44.0–48.0) | 44.0 (40.0–45.3) | 46.0 (44.0–48.0) |
| Viral load (log) | |||||
| Mean (SD) | 5.0 (2.9) | 7.2 (1.9) | 4.8 (2.9) | 5.8 (2.4) | 4.9 (3.0) |
| CK 18 serum level, U/L | |||||
| Mean (SD) | 206.6 (190.5) | 404.8 (236.6) | 175.7 (162.6) | 321.3 (304.1) | 177.5 (135.7) |
| Median (IQR) | 150.6 (95.8–242.9) | 378.5 (173.2–620.6) | 137.3 (87.5–197.7) | 177.8 (120.8–519.1) | 142.7 (88.8–214.4) |
| Significant inflammation, n (%) | |||||
| Yes (grade ≥ 7) | 22 (13%) | NA | NA | NA | NA |
| No (grade < 7) | 141 (87%) | NA | NA | NA | NA |
| Significant fibrosis, n (%) | |||||
| Yes (F ≥ 3) | 33 (20%) | NA | NA | NA | NA |
| No (F < 3) | 130 (80%) | NA | NA | NA | NA |
Abbreviations: ALK, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; IQR, interquartile range; NA, not applicable; xULN, upper limit of the normal range.
Elevated serum CK 18 levels in patients with significant inflammation and fibrosis
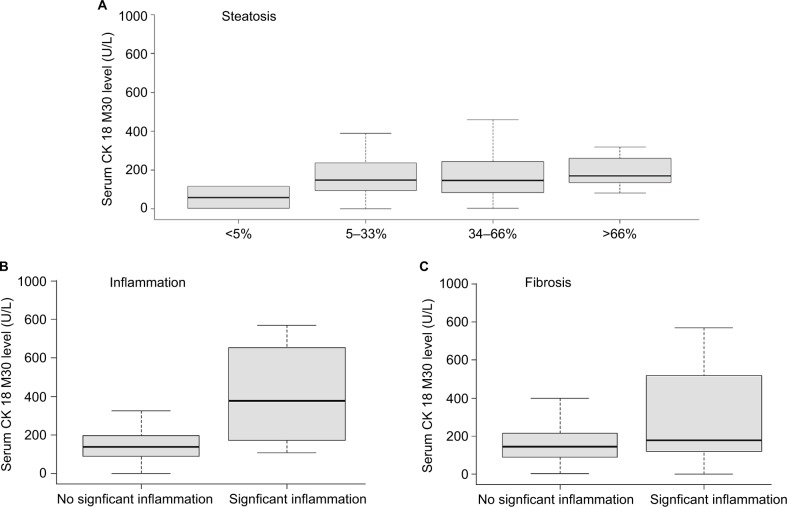
Figure 1 shows the serum CK 18 levels across patients when differentiated to steatosis grade, severity of inflammation, or fibrosis grade. When comparing steatosis grade, the difference in CK 18 serum levels was not significant (P > 0.05). However, there were significant differences in CK 18 levels among different grades of inflammation and fibrosis. Patients with significant inflammation had a higher CK 18 serum level than those with no significant inflammation (378.5 [IQR: 173.2–629.6] vs 137.3 [87.5–197.7], P < 0.05; approximately threefold increase). Similarly, patients who presented with significant fibrosis had a higher CK 18 level than those with no significant fibrosis (177.8 [IQR: 120.8–519.1] vs 142.7 [IQR: 88.8–214.4], P < 0.05; 1.25-fold increase). Correlation of CK 18 serum levels was analyzed with several other clinical parameters recorded in this cohort (Table 2). In terms of biochemical parameters, the strongest correlation was with alanine aminotransferase (ALT) and aspartate aminotransferase (AST; both r = 0.52; P < 0.001), followed by a negative correlation with albumin (r = −0.24, P < 0.05) and a positive correlation with viral load (log) (r = 0.19, P < 0.05). No correlation with either age or body mass index (BMI) was found (P > 0.05). Among the histological parameters, CK 18 correlated best with the grade of inflammation (r = 0.37; P < 0.001), followed by the grade of fibrosis (r = 0.18; P < 0.05). No correlation with steatosis was found (P > 0.05). Through multivariate analyses, adjusting for age, BMI, viral load (log), ALT level, ASL level, and albumin level, it was demonstrated that CK 18 serum levels can act as an independent predictor of the presence of significant inflammation (P < 0.05), but not for significant fibrosis (P > 0.05).
Figure 1.
Boxplots of serum CK 18 levels in relation with steatosis, inflammation, and fibrosis in in CHB patients.
Notes: (A) No differences across grades of steatosis (P>0.05); (B) significant differences between significant and no significant inflammation (P<0.05); (C) significant differences between significant and no significant fibrosis (P<0.05).
Table 2.
Correlation of clinical parameters with CK 18
| Parameters | ρ | P-value |
|---|---|---|
| Age | 0.04 | 0.603 |
| BMI | 0.11 | 0.183 |
| ALT | 0.52 | <0.0001 |
| AST | 0.52 | <0.0001 |
| Albumin | −0.24 | 0.015 |
| Viral load (log) | 0.19 | 0.017 |
| Steatosis | 0.07 | 0.368 |
| Inflammation | 0.37 | <0.0001 |
| Fibrosis | 0.18 | 0.020 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index.
Predictive value of CK 18 for significant inflammation
We then assessed the potential value of CK 18 as a diagnostic tool by estimating AUROC, sensitivity, and specificity. The AUROC of CK 18 for predicting significant inflammation was 0.81 (95% CI: 0.72–0.91; Figure 2). Sensitivity, specificity, positive/negative predictive values, and LR+/LR− are summarized in Table 3. Of note, all these values were calculated according to the optimal cutoff points of CK 18 levels: 243.0 U/L. Although CK 18 had a low sensitivity of 68% and a very low positive predictive value of 37%, it showed a high specificity of 82% and a high negative predictive value up to 94%.
Figure 2.
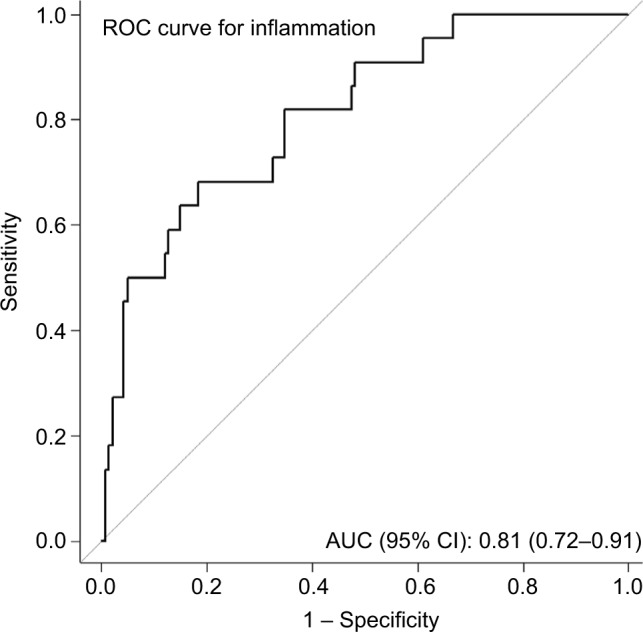
Predictive value of CK 18 for significant inflammation.
Notes: We assessed the potential value of CK 18 as a diagnostic tool by estimating AUC, sensitivity, and specificity. The AUC of CK 18 for predicting significant inflammation was 0.81 (95% CI: 0.72–0.91).
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Table 3.
Performance of serum CK 18 levels for the diagnosis of significant inflammation and significant fibrosis
| Parameter | Cutoff value (U/L) | Sensitivity (%) | Specificity (%) | PPV | NPV | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Significant inflammation | 243.0 | 68 | 82 | 37 | 94 | 3.9 | 0.4 |
Abbreviations: LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Discussion
The contemporary of biomedical literature supports the notion that CK18-Asp396 serum levels adequately reflect hepatocyte apoptosis.32 In our study, we exploited this notion to evaluate hepatocyte apoptosis in CHB, although we did not assess apoptosis directly. The study demonstrated, however, that CHB is indeed associated with CK18 release into the serum. As CK18 levels showed strong correlations with significant inflammation and fibrosis, with liver enzymes but not with steatosis, our results support the notion of CK18 being a marker for hepatocyte apoptosis. Especially, interesting are our results suggesting a diagnostic value of CK 18 serum levels for the identification of significant inflammation of the liver in CHB patients. The strong correlation with inflammatory responses suggests that hepatocyte apoptosis may serve as a strategy to limit the size of the chronic hepatitis B (HBV)-infected compartment, a notion possibly supported by the observation that viral load shows only weak correlation with CK18-Asp396 serum levels, possibly reflecting the functional effects of compartment size reduction. Future studies are necessary to substantiate this idea.
A previously published study suggested a combination of routine tests as noninvasive markers for liver inflammation and fibrosis.7 In that report, a prediction model was suggested for the identification of significant liver inflammation combining age, HBV DNA levels, AST, and albumin. However, in the current study, CK 18 serum level was the single independent predictor based on our multivariate analysis. In terms of significant inflammation, CK 18 serum level was regarded as a good predictor with an 82% specificity and a 68% sensitivity. The apparent superiority of CK18-Asp396 serum level determination over other noninvasive markers further highlighted the intimate connection between CHB-associated inflammation and hepatocyte apoptosis.
The diagnostic value of CK 18 serum levels for the identification of significant inflammation in viral infection-associated chronic liver disease corresponds well with the findings of Bae et al.21 This study reported a high specificity of 89% for CK 18 serum levels and a lower sensitivity of 45% for CHB patients, but also reported that the combination of CK 18 with AST yielded a much higher specificity up to 96% in their study, although simultaneously decreasing sensitivity to 38%.21 As a liver enzyme, AST is more representative of hepatocyte death in general rather as indicating hepatocyte apoptosis per se. In spite of a strong correlation between CK 18 and AST observed in our study, AST was not an independent predictor, suggesting that hepatocyte apoptosis and necrosis is a defining property in CHB.
Considering significant fibrosis, CK 18 serum levels were previously reported as a useful predictor of this process in NAFLD patients,10,23 and a correlation of CK 18 serum levels with fibrosis stage in CHB is supported by the observations of Sumer et al.24 Similar to our findings, these authors detect increased CK 18 serum levels when significant fibrosis is present. Our data, however, indicate that CK 18 serum level cannot predict significant fibrosis independently, which also contrasts a recent study showing that CK 18 serum levels work independently as a predictor of the presence of significant fibrosis (F ≥ 3).10 Generally speaking, we feel that hepatocyte apoptosis is unlikely to have a causal relationship with the fibrosis process, and in potential agreement the Rosso et al’s study presented a low AUROC value (0.61) for fibrosis with a sensitivity of 88% and a specificity of 38%.10 As a matter of fact, the beneficial role of apoptosis was ever reported in pancreatitis disease.33 Indeed, transient elastography,34 measuring liver stiffness, was found to be a better predictor than CK 18 serum levels. To obtain a more accurate prediction for the presence of significant fibrosis, both markers, transient elastography, and CK 18 serum levels were combined. Unfortunately, the combination performed not better than the transient elastography alone. In conclusion, we feel that CK 18 may have only limited value for the identification of significant fibrosis.
Conclusion
Our study has further demonstrated the predictive value of CK 18 serum levels as a noninvasive marker for the presence of significant liver inflammation in CHB. In addition, the potential combination with other noninvasive markers might improve the total performance, especially the sensitivity. With regard to the identification of significant fibrosis, we considered the limited diagnostic value of CK 18. Further study is required to identify more reliable and efficient noninvasive tool. Finally, our results support the notion that hepatocyte apoptosis has an important functionality in CHB pathogenesis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz B, Koklu S, Buyukbayram H, et al. Chronic hepatitis B associated with hepatic steatosis, insulin resistance, necroinflammation and fibrosis. Afr Health Sci. 2015;15(3):714–718. doi: 10.4314/ahs.v15i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang TJ, Kwekkeboom J, Laman JD, et al. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10(3):159–167. doi: 10.1046/j.1365-2893.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 4.Thabet K, Chan HL, Petta S, et al. The MBOAT7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology. 2017;65(6):1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 5.Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221–239. doi: 10.2147/CEG.S62831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohamadnejad M, Montazeri G, Fazlollahi A, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol. 2006;101(11):2537–2545. doi: 10.1111/j.1572-0241.2006.00788.x. [DOI] [PubMed] [Google Scholar]
- 8.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32(3):477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37:400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 10.Rosso C, Caviglia GP, Abate ML, et al. Cytokeratin 18-aspartate396 apoptotic fragment for fibrosis detection in patients with nonalcoholic fatty liver disease and chronic viral hepatitis. Dig Liver Dis. 2016;48(1):55–61. doi: 10.1016/j.dld.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Janssen HL, Higuchi H, Abdulkarim A, Gores GJ. Hepatitis B virus enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity by increasing TRAIL-R1/death receptor 4 expression. J Hepatol. 2003;39(3):414–420. doi: 10.1016/s0168-8278(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Vilchez S, Lara-Pezzi E, Trapero-Marugan M, Moreno-Otero R, Sanz-Cameno P. The molecular and pathophysiological implications of hepatitis B X antigen in chronic hepatitis B virus infection. Rev Med Virol. 2011;21(5):315–329. doi: 10.1002/rmv.699. [DOI] [PubMed] [Google Scholar]
- 13.Rasch S, Algul H. A clinical perspective on the role of chronic inflammation in gastrointestinal cancer. Clin Exp Gastroenterol. 2014;7:261–272. doi: 10.2147/CEG.S43457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakim MS, Spaan M, Janssen HL, Boonstra A. Inhibitory receptor molecules in chronic hepatitis B and C infections: novel targets for immunotherapy? Rev Med Virol. 2014;24(2):125–138. doi: 10.1002/rmv.1779. [DOI] [PubMed] [Google Scholar]
- 15.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40(5):403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 16.Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214(1):1–9. doi: 10.1016/j.canlet.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Ljumovic D, Diamantis I, Alegakis AK, Kouroumalis EA. Differential expression of matrix metalloproteinases in viral and non-viral chronic liver diseases. Clin Chim Acta. 2004;349(1–2):203–211. doi: 10.1016/j.cccn.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Yang ZH, Yang SX, Qin CZ, Chen YX. Clinical values of elevated serum cytokeratin-18 levels in hepatitis: a meta-analysis. Hepat Mon. 2015;15(5):e25328. doi: 10.5812/hepatmon.15(5)2015.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eren F, Yilmaz Y, Kose S, et al. Caspase-cleaved fragments of cytokeratin 18 in patients with chronic hepatitis B. Clin Chim Acta. 2010;411(23–24):2029–2032. doi: 10.1016/j.cca.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Han T, Gao YT, Jing L, Ma Z. The expression of serum M30 and M65 in chronic hepatitis B patients with non-alcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2015;19(21):4123–4129. [PubMed] [Google Scholar]
- 21.Bae CB, Kim SS, Ahn SJ, et al. Caspase-cleaved fragments of cytokeratin-18 as a marker of inflammatory activity in chronic hepatitis B virus infection. J Clin Virol. 2013;58(4):641–646. doi: 10.1016/j.jcv.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Jazwinski AB, Thompson AJ, Clark PJ, Naggie S, Tillmann HL, Patel K. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J Viral Hepat. 2012;19(4):278–282. doi: 10.1111/j.1365-2893.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with nonalcoholic fatty liver disease. J Hepatol. 2014;60(1):167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Sumer S, Aktug Demir N, Kolgelier S, et al. The clinical significance of serum apoptotic cytokeratin 18 neoepitope M30 (CK-18 M30) and matrix metalloproteinase 2 (MMP-2) levels in chronic hepatitis B patients with cirrhosis. Hepat Mon. 2013;13(6):e10106. doi: 10.5812/hepatmon.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 27.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer WP, van der Meer AJ, Boonstra A, et al. The impact of PNPLA3 (rs738409 C>G) polymorphisms on liver histology and long-term clinical outcome in chronic hepatitis B patients. Liver Int. 2015;35(2):438–447. doi: 10.1111/liv.12695. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz Y, Dolar E, Ulukaya E, et al. Elevated serum levels of caspase-cleaved cytokeratin 18 (CK18-Asp396) in patients with nonalcoholic steatohepatitis and chronic hepatitis C. Med Sci Monit. 2009;15(4):CR189–CR193. [PubMed] [Google Scholar]
- 30.Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80(6):1618–1625. doi: 10.1093/ajcn/80.6.1618. [DOI] [PubMed] [Google Scholar]
- 31.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18 – the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30(11–12):1103–1109. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser AM, Saluja AK, Lu L, Yamanaka K, Yamaguchi Y, Steer ML. Effects of cycloheximide on pancreatic endonuclease activity, apoptosis, and severity of acute pancreatitis. Am J Physiol. 1996;271(3 pt 1):C982–C993. doi: 10.1152/ajpcell.1996.271.3.C982. [DOI] [PubMed] [Google Scholar]
- 34.Verveer C, Zondervan PE, ten Kate FJ, Hansen BE, Janssen HL, de Knegt RJ. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012;32(4):622–628. doi: 10.1111/j.1478-3231.2011.02663.x. [DOI] [PubMed] [Google Scholar]