Abstract
Background
Chronic knee pain from osteoarthritis (OA) is common in the aging and the obese population. Radiofrequency ablation of the genicular nerves has been introduced as a potential surgery-sparing treatment for chronic knee pain from OA, yet only two outcome studies have been published and optimal patient selection for this procedure has not been established.
Objectives
We describe a standardized protocol for selecting patients for cooled radiofrequency ablation (C-RFA) of the genicular nerves, as well as the clinical outcomes of four patients ages 63-65 years.
Methods
The threshold for selection based on diagnostic genicular nerve block was ≥ 80% pain reduction. Following successful block, C-RFA of the genicular nerves was performed. Outcomes included pain, function, analgesic medication use, opioid use, and progression to total knee arthroplasty at a minimum of 6 month follow up.
Results
C-RFA of the genicular nerves after using the described selection protocol resulted in > 90% pain reduction, improved function and avoidance of surgery at 6 months in all four cases. All opioid and analgesic medication use decreased or was unchanged in all cases. No serious adverse events occurred.
Conclusions
The accompanying case series suggests that this protocol is deserving of randomized, prospective study.
Keywords: Osteoarthritis, Knee, Chronic Pain, Radiofrequency Catheter Ablation, Outcome Assessment (Health Care)
1. Background
Osteoarthritis (OA) of the knee joint is a common cause of pain and functional impairment, as well as opioid use in approximately 40% of patients with this condition (1, 2). Initial treatment of this condition includes weight loss, physical therapy, oral analgesics, and intra-articular steroid or viscosupplementation injections (3). If conservative treatment fails, total knee replacement (TKA) is traditionally offered, yet TKA is associated with multiple perioperative morbidities (4). Additionally, many patients with knee OA are not surgical candidates due to co-morbidities (5). Other patients simply do not want an elective, invasive surgery. Until recently, these patients have had sub-optimal treatment options, including chronic opioid pain management (6), and typically continue to experience significant pain and disability (7). Radiofrequency ablation (RFA) of the genicular nerves has recently emerged as a treatment option in such situations (8-12). Preliminary outcomes for genicular nerve RFA are promising (8-12).
2. Objectives
However, unlike medial and lateral branch RFA (13-15) established criteria for patient selection prior to RFA of the genicular nerves does not exist. The only randomized prospective study to date used a selection criterion of ≥ 50% pain reduction following diagnostic genicular nerve block, and subsequently, less than 60% of subjects experienced a clinically significant reduction in pain (9). Here, we describe a systematic, more rigorous protocol for selecting patients for cooled radiofrequency ablation (C-RFA) of the genicular nerves, including a threshold requirement of ≥ 80% pain reduction following diagnostic genicular nerve block. In addition, we present the clinical outcomes of four sequential patients with chronic refractory knee pain from OA who underwent the genicular C-RFA procedure and following this selection protocol, as a call for further randomized, prospective study.
3. Methods
Patients who presented to our pain management center with chronic knee pain consistent in character with osteoarthritis confirmed by imaging, who had failed conservative management including intra-articular injection therapy and were either candidates for TKA but wished to avoid surgery, or were not eligible for TKA due to medical co-morbidities, were offered genicular nerve diagnostic blocks with the possibility of genicular nerve C-RFA. All patients underwent one set of diagnostic blocks. A positive response to diagnostic blockade was defined as ≥ 80% reduction in baseline pain, concordant with the local anesthetic duration of action, while weight bearing and walking. This 80% threshold was based on recommendations from the spine intervention society (SIS) guidelines for facet joint denervation (15). Patients were then asked to rate their percentage reduction in knee pain while performing ambulation, squatting and any other maneuvers that typically provoke their pain during the next 30 minutes in the office. They were additionally asked to log percent pain reduction over the following 6 hours and call to report the results on the following business day. In the accompanying series of patients, all who reported ≥ 80% reduction in pain with this protocol underwent C-RFA of the genicular nerves.
3.1. Diagnostic Genicular Nerve Block Procedure
The patient was positioned supine on a fluoroscopy table with a bolster to provide 30 - 40 degrees of flexion in the treated knee joint. The foot and ankle were secured to the fluoroscopy table with wide, durable tape, to stabilize the leg during the procedure. Using a 25-gauge needle, a skin wheal of 1 - 2 mL of 1% lidocaine was used for superficial local anesthesia in order to avoid spread to the genicular nerves (a potential cause of false-positive block results). A 25-gauge 2.5 - 3.5 inch Whitacre needle was placed at 3 unique anatomic sites to block the following neural structures: the superior lateral, the superior medial, and the inferior medial genicular nerves. The superior lateral genicular nerve is located at the confluence of the lateral femoral shaft and the lateral femoral condyle (in the anteroposterior (A - P) plane) and at the midpoint of the femur (in the lateral plane). The superior medial genicular nerve site is located at the confluence of the medial femoral shaft and the medial femoral condyle (in the A - P plane) and at the midpoint of the femur (in the lateral plane). The inferior medial genicular nerve site was located at the confluence of the medial tibial shaft and the tibial flare (in the A - P plane) and the midpoint of the tibia (in the lateral plane) (16). Accurate and precise needle placement was confirmed using fluoroscopy in both the A - P and lateral planes, taking extra care to ensure that the condyles of the femur were superimposed over one another during lateral imaging to eliminate obliquity. Needle placement sites are shown in Figure 1. At each needle site, 1.0 mL of 2% lidocaine was injected in order to anesthetize each genicular nerve.
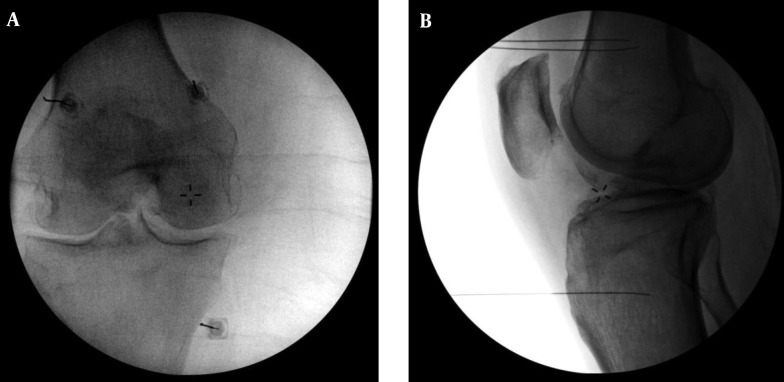
Figure 1. A, Anterior/Posterior and B, Lateral Fluoroscopic Images of the Final Needle Positions During Diagnostic Block Genicular Nerve Blocks are Shown.
The superior lateral site is identified at the confluence of the lateral femoral shaft and the lateral femoral condyle in the A - P plane and the midpoint of the femur in the lateral plane. The superior medial site is identified at the confluence of the medial femoral shaft and the medial femoral condyle in the A - P plane and the midpoint of the femur in the lateral plane. The inferior medial site is identified at the confluence of the medial tibial shaft and the tibial flare in the A - P plane and the midpoint of the tibia in the lateral plane. Of note, the lateral view (1B) shows some obliquity in which the femoral condyles are not perfectly superimposed. This is ideally avoided. The lateral view during electrode placement (shown in Figure 2B) represents a more ideal view with no obliquity.
3.2. Genicular Nerve Radiofrequency Ablation Procedure
Patient positioning and monitoring was identical to the diagnostic genicular nerve block procedure. Conscious sedation (midazolam 1 - 2 mg IV and/or fentanyl 25 - 100 mcg IV) and supplemental nasal cannula oxygen were administered. Skin and soft tissues were anesthetized with 1 - 2 mL of 1% lidocaine at each of the 3 anatomic sites for RFA, and a 50 or 75 millimeter 17-gauge introducer needle was placed to lesion the superior lateral, superior medial, and inferior medial genicular nerves. Once the introducer needle was placed, the 18 g internally cooled, 4 mm active tip RFA electrode (Coolief, Halyard Health, Alpharetta, GA) was placed into the introducer needle and positioning was verified with A-P and lateral fluoroscopic views. After motor nerve activity was ruled out with testing at 2 Hertz at 1 mA, 1 mL of 2% lidocaine was injected through the introducer needles to anesthetize the region prior to thermal ablation. Each target was sequentially lesioned for 2 minutes and 30 seconds at a set temperature of 60 degrees C, which imparts a tissue temperature of 77 - 80°C surrounding the electrode (17). Electrode position is shown in Figure 2.
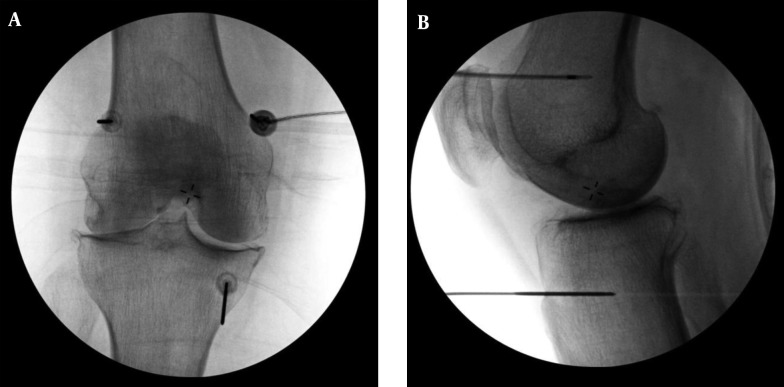
Figure 2. A, Anterior/Posterior and B, Lateral Fluoroscopic Images of the Final Electrode Positions During C-RFA of the Genicular Nerves are Shown.
Specific final electrode positions are identical to the final needle positions described in the Figure 1 caption.
3.3. Clinical Outcomes Reported
Pain reduction on a numeric rating scale (NRS) and functionality by self-report were assessed, as well as changes in analgesic and opioid medication use were measured using the medication quantification scale III (MQS3), as previously described (18-20).
3.4. Case Presentations
3.4.1. Case 1
A 65-year-old obese man, (BMI 41) presented with progressive left knee pain of more than 5 years duration. Radiographs of the knee showed Kellgren-Lawrence grade 3 medial compartment OA. He reported 9/10 on the NRS, worsened by range of motion and weight bearing. He used a cane for ambulation. Non-steroidal anti-inflammatory medications, three intra-articular steroid injections and physical therapy provided only modest transient pain reduction. Due to anemia of unknown etiology, TKA was contraindicated per orthopedic surgery. On examination, he demonstrated reduced knee range of motion in flexion, medial joint line tenderness to palpation, and an antalgic gait.
3.4.2. Case 2
A 63-year-old morbidly obese man (BMI 43) with disabling bilateral knee pain presented after several years of symptom progression following arthroscopic repair of a torn right meniscus. Radiographs of the knees confirmed tri-compartmental knee OA, Kellgren-Lawrence grade 3 on the left and grade 4 on the right. He reported 5/10 pain on the NRS, worsened with knee flexion. He noted limited ability to ambulate and perform stair climbing. Six periodic intra-articular steroid injections and physical therapy previously provided symptom relief, but were no longer effective. He had avoided use of opioids but was taking ibuprofen up to 1200 mg daily. Physical examination revealed reduced end-range flexion range of motion bilaterally, medial and lateral knee joint line tenderness bilaterally, and antalgic gait.
3.4.3. Case 3
A 66-year-old woman (BMI 35) presented with 3 years of bilateral knee pain due to previously diagnosed OA. Radiographs of the knee showed Kellgren-Lawrence grade 3 and 4 medial compartment OA, on the left and right sides, respectively. She reported 7/10 pain in the right knee and 5/10 pain in the left knee on the NRS, worsened with prolonged walking. Her pain had been managed with tramadol 100 mg daily. NSAIDs were contraindicated due to clopidogrel use for coronary artery disease (CAD). She had undergone physical therapy and five intra-articular steroid injections with diminishing benefit. She refused bilateral TKA due to concerns about her cardiac disease. Physical exam was remarkable for bilateral valgus deformities of the knees with decreased end-range flexion range of motion, medial and lateral joint line tenderness, and antalgic gait with use of a cane.
3.4.4. Case 4
A 64 year-old man with Parkinson’s disease (BMI 24) and a remote history of right meniscectomy presented with progressive right knee pain. Radiographs of the knee demonstrated Kellgren-Lawrence grade 3 medial compartmental OA. He reported 5/10 pain on the NRS, worse with prolonged sitting. He had undergone four intra-articular steroid injections and physical therapy without sustained benefit. He was taking ibuprofen 200 mg as needed 1 - 2 times weekly. Physical exam was significant for crepitus with right knee range of motion, medial joint line tenderness, and an antalgic, shuffling Parkinsonian gait, for which he used a cane.
4. Results
Clinical outcomes of the above cases are shown in Table 1. All four patients reported 80% - 100% improvement in knee pain at 6 - 12 month follow up. All patients reported improved daily function, including walking and climbing stairs. One of the two patients taking opioids reduced use. Three patients had improved MSQ3 scores, while all four showed improved MSQ3 scores when excluding pain medications taken for an unrelated pain condition (low back and radicular pain in Case 1). There were no complications associated with the procedure in any of the four cases.
Table 1. Summary of Case Presentations.
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 65 | 63 | 66 | 64 |
| Sex | M | M | F | M |
| BMI | 41 | 43 | 35 | 24 |
| Unilateral (U) vs. Bilateral (B) RFA | U | B | B | U |
| Baseline NRS (Right/Left, if applicable) | 6 | 5/4 | 7/5 | 3 |
| Baseline MQS3/MEq | 32.1/64 | 8/0 | 4.8/10 | 4/0 |
| Percent reduction in pain with test block | 100 | 100/100 | 86/100 | 100 |
| Percent reduction in pain 3 month post-RFA | 100 | 90/90 | 80/50 | 90 |
| Percent reduction in pain 6 months post-RFA | 100 | 90/90 | 85/80 | 90 |
| Percent reduction in pain 9 months post-RFA | 90 | 80/80 | N/A | N/A |
| Reduction in MQS3 score at 6 months post-RFA | -6.9a | 8 | 4.8 | 4 |
| Reduction in Morphine equivalent consumption at 6 months post-RFA | -8a | N/A | 10 | N/A |
| Self-Reported Functional Change | Initially improvement walking with elimination of cane use and improved squat transfers | Improved prolonged ambulation and stair climbing | Improved transfers from sitting and prolonged ambulation | Improved prolonged standing and ambulation |
| Current Surgical Status (TKA or None) | None | None | None | None |
aRise in MSQ3 score related to low back and radicular pain, not knee pain.
5. Discussion
We describe a systematic and rigorous patient selection protocol for genicular RFA to include a threshold of ≥ 80% pain reduction following diagnostic genicular nerve blocks prior to ablation. This protocol is more stringent than that which has been previously reported (9). Using this proposed protocol, we report improved pain and function in four consecutive patients who underwent uni- or bilateral C-RFA for treatment of chronic knee pain from primary OA that had been refractory to non-surgical treatment. All four patients reported 80-100% improvement in knee pain, function, and analgesic medication use at 6 - 12 month follow-up.
While there are five publications regarding outcomes of genicular RFA (8-12), only one describes a patient selection protocol for patients with chronic pain due to primary osteoarthritis of the knee joint. A randomized controlled trial of genicular RFA by Choi et al used a threshold of ≥ 50% reduction in pain after diagnostic genicular nerve block for a minimum of 24 hours. This threshold is low compared to guidelines for other joint denervation selection protocols (13-15) and the duration of > 24-hour improvement in pain is inconsistent with the duration of action of any local anesthetic. Despite this relatively loose inclusion criteria, they reported statistically significant reduction in pain scores in the treatment group (n = 19) at 4 and 12 weeks as compared to the sham-control group, and a 59% treatment success rate, defined as ≥ 50% pain reduction at 12 weeks (9). Of note, the mean BMI in their study population was only 26, far lower than what is typical in the chronic knee pain population with primary osteoarthritis, which limited the generalizability of their results. Comparably, we report excellent clinical outcomes in this case series when a threshold of ≥ 80% pain reduction with diagnostic nerve blocks is used for patient selection. Indeed, further study is needed to test our hypothesis in a larger, prospective study, and to identify other patient factors that could predict treatment success (e.g. age, BMI, degree of knee joint degeneration, co-morbidities). These are well described for facet joint and sacroiliac joint denervation (13-15, 19, 21).
The optimal number of genicular branches that need to be ablated to achieve successful outcomes is also needed. The literature to date supports ablation of the superior medial, superior lateral and inferior medial genicular nerves (8-12), and the inferior lateral branch is avoided due to its proximity to the common peroneal nerve and risk of motor neuron injury and foot drop (16). The need to ablate the intermediate genicular nerve, thought to provide afferent sensation to the patellofemoral compartment of the knee joint and capsule (16), has been debated, but has yet to be investigated.
Other procedural factors beg further investigation as well. In our case series, we used conscious sedation for genicular nerve ablation, but not for diagnostic blockade. Given the size of the introducer needles (17 g) and the proximity of needle placement near the periosteum of a painful, arthritis, and often enflamed joint, highly innervated with afferent pain fibers (22) the ablation procedure can be painful. Without conscious sedation, higher volumes of local anesthesia would be needed, but could cause false negative motor nerve testing and pose additional procedural risks to the patient.
Anatomic studies show that the genicular nerves are not within the proximity of a vascular network (16), and thus, we did not use contrast dye injections to rule out intravascular local anesthetic spread during diagnostic blocks in this series. It is theoretically possible that use of contrast could minimize the rate of false negative genicular nerve blocks, but this requires hypothesis testing.
Finally, it must be acknowledged that genicular nerve ablation using C-RFA is an emerging procedure. No adverse events related to this procedure using thermal or cooled RFA have been reported in the published literature to date (8-12). However, post-procedural neuritis/deafferentation pain is a theoretical concern, as this is a known adverse event associated with RFA of the medial branch and sacral lateral branch nerves (23-25). Third degree skin burn has been reported with the use of C-RFA to denervate a thoracic medial branch nerve in a patient with a very thin body habitus (26). While Charcot joint is unlikely given incomplete denervation of the knee when ablating only the superiomedial, superiolateral, and inferomeidal genicular nerves, no long-term investigation has been reported to confirm or refute this possibility.
5.1. Conclusions
We present a stringent patient selection protocol for C-RFA of the genicular nerves for the treatment of chronic refractory knee pain due to osteoarthritis. As this procedure becomes increasingly popular, stringent selection criteria will be vital to maximize clinical outcomes, a hypothesis that requires validation in a larger, prospective clinical trial.
References
- 1.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 2.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489–95. doi: 10.1002/acr.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruyere O, Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44(3):253–63. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479–85. doi: 10.1302/0301-620X.96B4.33209. [DOI] [PubMed] [Google Scholar]
- 5.Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg. 2010;80(9):595–9. doi: 10.1111/j.1445-2197.2010.05396.x. [DOI] [PubMed] [Google Scholar]
- 6.McCartney CJ, Nelligan K. Postoperative pain management after total knee arthroplasty in elderly patients: treatment options. Drugs Aging. 2014;31(2):83–91. doi: 10.1007/s40266-013-0148-y. [DOI] [PubMed] [Google Scholar]
- 7.Imani F, Safari S. "Pain Relief is an Essential Human Right", We Should be Concerned about It. Anesth Pain Med. 2011;1(2):55–7. doi: 10.5812/kowsar.22287523.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeuchi M, Ushida T, Izumi M, Tani T. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12(4):546–51. doi: 10.1111/j.1526-4637.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi WJ, Hwang SJ, Song JG, Leem JG, Kang YU, Park PH, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152(3):481–7. doi: 10.1016/j.pain.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Bellini M, Barbieri M. Cooled radiofrequency system relieves chronic knee osteoarthritis pain: the first case-series. Anaesthesiol Intensive Ther. 2015;47(1):30–3. doi: 10.5603/AIT.2015.0003. [DOI] [PubMed] [Google Scholar]
- 11.Rojhani S, Qureshi Z, Chhatre A. Water-Cooled Radiofrequency Provides Pain Relief, Decreases Disability, and Improves Quality of Life in Chronic Knee Osteoarthritis. Am J Phys Med Rehabil. 2016 doi: 10.1097/PHM.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 12.Shen WS, Xu XQ, Zhai NN, Zhou ZS, Shao J, Yu YH. Radiofrequency Thermocoagulation in Relieving Refractory Pain of Knee Osteoarthritis. Am J Ther. 2016 doi: 10.1097/MJT.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfuss P, Halbrook B, Pauza K, Joshi A, McLarty J, Bogduk N. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976). 2000;25(10):1270–7. doi: 10.1097/00007632-200005150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13(3):383–98. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 15.April C, Bogduk N, Dreyfuss P, Endres S, Govind J, Kraft M, et al. In: Practice guidelines for spinal diagnostic and treatment procedures. Bogduk N, editor. San Francisco: International Spine Intervention Society; 2004. [Google Scholar]
- 16.Franco CD, Buvanendran A, Petersohn JD, Menzies RD, Menzies LP. Innervation of the Anterior Capsule of the Human Knee: Implications for Radiofrequency Ablation. Reg Anesth Pain Med. 2015;40(4):363–8. doi: 10.1097/AAP.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 17.Cosman ERJ, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med. 2014;15(12):2020–36. doi: 10.1111/pme.12566. [DOI] [PubMed] [Google Scholar]
- 18.McCormick ZL, Gagnon CM, Caldwell M, Patel J, Kornfeld S, Atchison J, et al. Short-Term Functional, Emotional, and Pain Outcomes of Patients with Complex Regional Pain Syndrome Treated in a Comprehensive Interdisciplinary Pain Management Program. Pain Med. 2015;16(12):2357–67. doi: 10.1111/pme.12817. [DOI] [PubMed] [Google Scholar]
- 19.McCormick ZL, Marshall B, Walker J, McCarthy R, Walega DR. Long-Term Function, Pain and Medication Use Outcomes of Radiofrequency Ablation for Lumbar Facet Syndrome. Int J Anesth Anesth. 2015;2(2) doi: 10.23937/2377-4630/2/2/1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallizzi M, Gagnon C, Harden RN, Stanos S, Khan A. Medication Quantification Scale Version III: internal validation of detriment weights using a chronic pain population. Pain Pract. 2008;8(1):1–4. doi: 10.1111/j.1533-2500.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SP, Hurley RW, Buckenmaier C3, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–88. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralston H3, Miller MR, Kasahara M. Nerve endings in human fasciae, tendons, ligaments, periosteum, and joint synovial membrane. Anat Rec. 1960;136:137–47. doi: 10.1002/ar.1091360208. [DOI] [PubMed] [Google Scholar]
- 23.Stolzenberg D, Gordin V, Vorobeychik Y. Incidence of neuropathic pain after cooled radiofrequency ablation of sacral lateral branch nerves. Pain Med. 2014;15(11):1857–60. doi: 10.1111/pme.12553. [DOI] [PubMed] [Google Scholar]
- 24.Lord SM, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: a caution. Neurosurgery. 1995;36(4):732–9. doi: 10.1227/00006123-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kornick C, Kramarich SS, Lamer TJ, Todd Sitzman B. Complications of lumbar facet radiofrequency denervation. Spine (Phila Pa 1976). 2004;29(12):1352–4. doi: 10.1097/01.brs.0000128263.67291.a0. [DOI] [PubMed] [Google Scholar]
- 26.Walega D, Roussis C. Third-degree burn from cooled radiofrequency ablation of medial branch nerves for treatment of thoracic facet syndrome. Pain Pract. 2014;14(6):154–8. doi: 10.1111/papr.12222. [DOI] [PubMed] [Google Scholar]