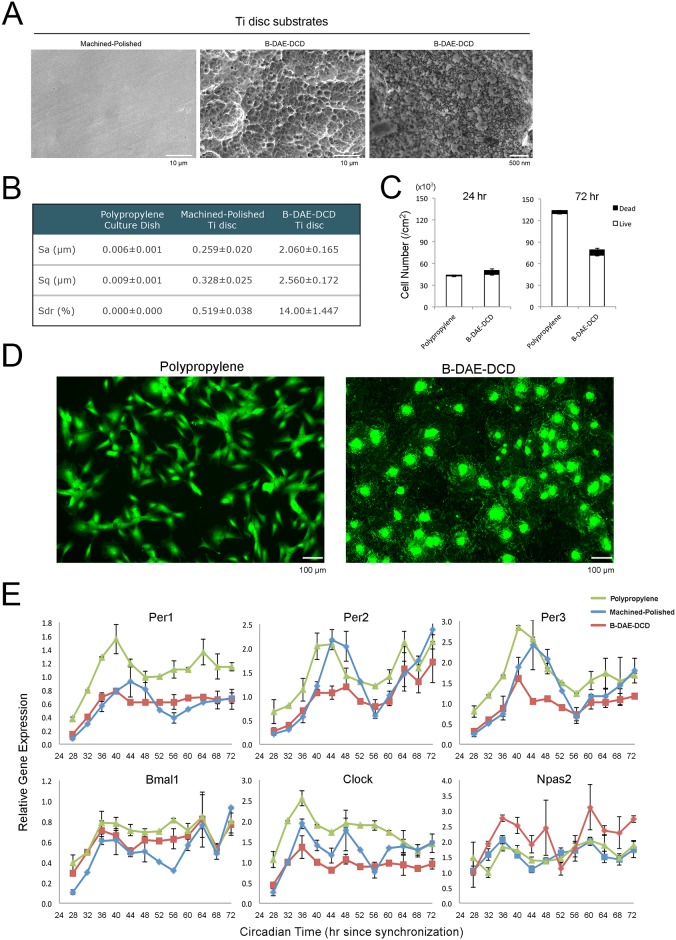
Fig 1. Titanium (Ti)-based biomaterials significantly modulated the expression patterns of circadian rhythm genes from human bone marrow stromal cells (BMSCs).
A. Scanning electron microscopy was used to characterize the surface of the Ti discs used in this study. The machined-polished Ti discs showed a smooth surface, whereas the Ti disc treated by sand-blasting followed by double acid etching and discrete calcium phosphate nanoparticle deposition (B-DAE-DCD) showed a complex surface topography at the micrometer and nanometer range. B. The surface topography was quantitatively evaluated by optical photometry (n = 3 in each group). The B-DAE-DCD surface was approximately 10x rougher than the machined-polished surface. C. Human BMSCs cultured on conventional polypropylene culture dishes (n = 4 per time point) or B-DAE-DCD Ti discs (n = 4 per time point) were synchronized by forskolin and exposed to 1 nM 1,25(OH)2D3 vitamin D-supplemented culture medium. The number of BMSCs was determined using a live/dead assay at 24 hours and 72 hours of culture. D. Calcein-stained live BMSCs cultured on polypropylene dishes maintained a fibroblastic morphology after 72 hours of culture. By contrast, the live BMSCs on the B-DAE-DCD discs were widely spread and made contacts with adjacent cells, resulting in the establishment of confluency. E. The steady state mRNA levels of circadian rhythm-related genes were determined by PCR every 4 hours starting from 24 hours to 72 hours after synchronization (n = 4 per time point in each group). PER1, PER2 and PER3 from human BMSCs cultured on polypropylene culture dishes and machined-polished discs exhibited a circadian expression pattern. When cultured on the B-DAE-DCD discs, the circadian expression pattern was diminished, while NPAS2 was upregulated compared to the polypropylene control.