Abstract
Background
Helicobacter pylori infection is strongly associated with gastric cancer occurrence. However, it is unclear whether eradication therapy reduces the risk of gastric cancer occurrence. We evaluated whether H. pylori eradication reduces the risk of primary gastric cancer by using both risk ratio (RR) and risk difference (RD).
Methods
Searches of PubMed, EMBASE, Google scholar, the Cochrane Library, and the Japan Medical Abstracts Society as well as those registered in databases of the Cochrane Central Register of Controlled Trials, metaRegister of Controlled Trials, ClinicalTrials.gov, controlled-trials.com, UMIN-CTR, JMACCT-CTR, and JAPIC-CTI between January 1965 and March 2017, supplemented with manual screening. Randomized controlled trials (RCTs) in which eradication therapy were implemented for the interventional group but not for the control group, and assessed the subsequent occurrence of primary gastric cancer as the main outcome. Two authors independently reviewed articles and extracted data. Integrated results for all data were presented as RR and RD.
Results
Seven studies met inclusion criteria. The reductions in risk of primary gastric cancer occurrence in terms of overall RR and RD were 0.67 (95% CI: 0.48 to 0.95) and -0.00 ([95% CI: -0.01 to 0.00]; number needed to treat: 125.5 [95% CI: 70.0 to 800.9]), respectively.
Conclusions
The effectiveness of H. pylori eradication therapy in suppressing the occurrence of primary gastric cancer was significant and comparable to that of previous studies in terms of the estimated RR. However, the estimated RD was slight and not statistically significant.
Introduction
Helicobacter pylori was designated a definite carcinogen of gastric cancer in 1994 by the International Agency for Research on Cancer (IARC) [1]. Infection of the gastric mucosa eventually leads to cancer via superficial gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia [2]. Take et al. confirmed reductions in gastric cancer occurrence in follow-up studies of patients with peptic ulcers who had received eradication therapy [3, 4]. Fukase et al. showed in a randomized controlled trial (RCT) that H. pylori eradication therapy following endoscopic treatment of early-stage gastric cancer reduced the occurrence of metachronous gastric cancer by approximately 30% [5].
Fuccio et al. conducted a meta-analysis of seven RCTs that had been published in 2009, and reported that H. pylori eradication significantly reduces primary gastric cancer by roughly 35% in patients with gastritis or precancerous lesions [6]. However, this analysis included an RCT, in which endoscopic resection of early-stage gastric cancer suppressed the occurrence of metachronous cancer [5], as well as a set of multiple articles for a single study [7]. Ford and Moayyedi re-examined these data when these studies were excluded, and found that although the effect size was comparable, the reduction was no longer significant [8]. Subsequently, Ford et al. conducted systematic reviews within Asia of gastritis or precancerous lesions triggered by H. pylori and demonstrated that gastric cancer occurrence may be prevented by eradication therapy, based on the results of six RCTs published in 2013 [9, 10]. In 2016, Lee, et al. updated the systematic review and showed a similar result [11].
Risk ratio (RR), a synonym for relative risk, means the ratio of two risks, usually of exposed and not exposed [12]. An RR of <1 means the event is less likely to occur in the interventional group than in the control group. Risk difference (RD), a synonym for attributable risk, means the difference of two risk, usually risk in the exposed minus risk in the unexposed. When the probability of primary endpoint of intervention group and placebo group is low, it is sometimes replaced by a large number in terms of risk ratio despite being slightly expressed by risk difference. These four systematic reviews [6, 9–11] only used the risk ratio (RR) as an integrative index. In risk communication, the ratio index is concerned to exaggerate the effect size compared to the absolute value or difference index [13, 14]. In based on the absolute value is inevitable for evaluating the size of the problem and is also useful for the deployment of policies. Although it has been repeatedly and strongly recommended to combine the risk difference and the risk ratio [15–19], it has been reported that the risk difference compared to the risk ratio is reported less [20–22]. Especially in the issue of primary prevention, in general, the absolute risk is small and the effect size of the intervention shown with the risk ratio will give an exaggerated impression.
This study aimed to re-examine the significance of H. pylori eradication therapy in suppressing gastric cancer by expanding the period and range of the literature search and by showing effect sizes with RR and risk difference (RD).
Methods
This systematic review and meta-analysis followed the PRISMA guidelines [23].
Literature search
We searched the literature for RCTs published between January 1965 and March 2017, using PubMed, EMBASE, Google scholar, and the Cochrane library as English literature databases. Considering the high prevalence of gastric cancer in Japan and the availability of Japanese research articles, we also searched the Japan Medical Abstracts Society database [24]. Search terms were as follows: Helicobacter pylori, gastric cancer, intestinal metaplasia, gastric atrophy, dysplasia, atrophic gastritis, and chronic gastritis (final search conducted in April 2017). Electronic search strategy for PubMed database was shown in Table 1.
Table 1. Electronic search strategy for PubMed database.
| Search (((((randomized controlled trial) OR (randomized controlled trials))) AND ((chronic gastritis) OR (gastritis) OR (atrophic gastritis) OR (dysplasia) OR (gastric atrophy) OR (gastric metaplasia) OR (intestinal metaplasia))) AND helicobacter pylori) AND gastric cancer Filters: Humans; English; Japanese |
A search was also conducted for clinical trials that had been registered by March 2017, using the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) [25], metaRegister of Controlled Trials (mRCT) [26], ClinicalTrials.gov [27], controlled-trials.com [28], University Hospital Medical Information Network in Japan Clinical Trials Registry (UMIN-CTR) [29], Center for Clinical Trials, Japan Medical Association Clinical Trial Registry (JMACCT-CTR) [30], and Japan Pharmaceutical Information Center Clinical Trials Information (JAPIC-CTI) [31] (final search conducted in April 2017). In addition, we searched for previous systematic reviews and meta-analyses on the same topic. After this, we read through all titles and abstracts of the following representative Japanese journals in gastroenterology: “I to Cho” (Stomach and Intestine, published by Igaku-Shoin Ltd., Tokyo), “Shokakinaishikyou” (Endoscopia Digestiva, published by Tokyo Igakusha Ltd., Tokyo), and “Rinsho Shokakinaika” (Clinical Gastroenterology, published by Nihon Medical Center Inc., Tokyo) (final search conducted in April 2017). We then read through the entire text of articles related to the present topic. In cases where the same study participants were observed for gastric cancer occurrence at different times, we used the research article with the longest follow-up period.
Finally, we checked for studies in which multiple articles resulted from a single study, wherein extended observations of the same research participants included in the initial phase of the study were reported after the observations from the initial study had been published. Specifically, we contacted corresponding authors via e-mail and asked whether a single study resulted in multiple articles [32]; we decided in advance that if there was no reply after one week, a reminder e-mail would be sent to the author, and that if there was still no reply, the follow-up would be discontinued.
We used the “PRISMA-2009-Checklist” to evaluate the quality of literature search and eligibility criteria for this systematic review and meta analysis in S1 Table.
Eligibility criteria
Inclusion criteria
Inclusion criteria were as follows: an RCT of H. pylori eradication therapy, or a study in which the occurrence of primary gastric cancer was subsequently followed up; an interventional group involving eradication therapy; a control group given “a placebo,” “no treatment (observation),” “a supplement,” or “an antacid containing a proton pump inhibitor (PPI),” none of which eradicated H. pylori; published in either English or Japanese; evidence of H. pylori infection demonstrated by a biochemical, serological, bacteriological, or histological method; and absence of gastric cancer as determined in advance by upper gastrointestinal endoscopy. Inclusion did not depend on the presence or absence of symptoms during participation in the study.
Exclusion criteria
Exclusion criteria were as follows: administration of eradication therapy in the control group; eradication therapy prior to group allocation; studies in which gastric cancer occurrence was not measured; studies that tracked whether metachronous cancer occurred after endoscopic therapy (endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD)) for primary cancer; animal studies; basic medical research; pathological research; reviews; and guidelines.
Main outcome measure
We designated primary gastric cancer occurrence as the outcome, regardless of whether the measure was the primary or secondary outcome in each study.
Data extraction
Based on eligibility criteria, two authors (T.S. and T.N.) independently checked the titles and summaries of all articles, searched and determined which were appropriate for inclusion. The other co-authors checked every process, and any issues that arose were resolved by discussion. The entire text of included articles was read, and the following information was extracted: year of publication; publication format; protocol; number of participating facilities in each study; subject characteristics; study country; language of publication; presence or absence of upper gastrointestinal symptoms at study initiation; method of eradication therapy; type of treatment in the control group; whether or not secondary eradication therapy was performed; treatment period; method of assessing H. pylori infection; number of times successful eradication was confirmed; primary and secondary outcomes; description of changes in gastric mucosa following eradication therapy; mean age; whether or not a difference was observed between the interventional and control groups; number of subjects; gastric cancer occurrence; histological type of gastric cancer; site of gastric cancer; degree of gastric cancer progression; gastric histological features at study initiation; histological assessment using updated Sydney System scores before and after eradication therapy [33]; proportion of subjects showing either intestinal metaplasia or dysplasia; clearly defined rationale for specifying the follow-up period; follow-up period; H. pylori eradication rate; drop-out rate; mean time to cancer occurrence; and time between endoscopies. Atrophic gastritis, intestinal metaplasia, and dysplasia were defined as precancerous lesions. We also noted whether there were multiple articles for a single study. If differences arose regarding details of the extracted data, all authors continued discussions until a consensus was achieved.
Quality assessment for primary trials
The quality of primary trials was assessed as described by Jadad et al [34]. This method assesses whether the trial is randomized, the appropriateness of randomization if present, whether the trial is double-blinded, the appropriateness of double-blinding if present, and withdrawals/dropouts, using a score of 0 or 1 for each item. Total scores thus range from 0 to 5. A high quality trial in this meta-analysis was defined by a Jadad score of ≥3 points. The GRADE system was used to evaluate the risk of bias of each trial used in this meta-analysis [35]. An RCT was considered high quality if three or more of the six domains for assessing risk of bias were adequate. Ultimately, the quality of a study was determined by either the Jadad score or risk of bias.
Statistical analysis
In four previous meta-analyses [6, 9–11], the pooled RR was used as the main index. Because this index tends to overestimate the effect, we first used the pooled RD and then the pooled RR, after which the results were presented together. The RD was calculated as risk in the interventional group minus that in the control group. The RD described the absolute change in risk that was attributable to the intervention. In other words, if the intervention had an identical effect to the control, RD would be 0. If it reduced the risk, the risk difference would be less than 0; if it increased the risk, the RD would be bigger than 0. The RD ranged from -1 to 1. A “+” sign indicated that treatment was favored, while a “-” sign indicated that the control was favored. Afterwards, the weighted pooled estimates were calculated for binary data. A fixed-effect model weighted by the Mantel-Haenszel (M-H) method was used to pool RD [36], followed by a test of homogeneity. Homogeneity among trials was assessed using the I2 test [37]. If the hypothesis of homogeneity was rejected, a random-effect model using the DerSimonian-Laird method was employed [38]. The potential for publication bias was examined by the funnel plot method [39], and the statistical significance of differences was evaluated in accordance with the methods of Begg or Egger [40, 41]. Given the observed risk difference, the number of patients that need to be treated (NNT) to prevent one adverse effect was also used as a measure of treatment effect; computationally speaking, NNT = 1/RD. Furthermore, the impact of eradication therapy, compared with placebo or no treatment, was expressed as a relative risk of occurrence of gastric cancer with 95% confidence intervals (CIs). All statistical analyses were performed with STATA statistical software version 14 [42]. Results are expressed as means and 95% CIs, unless indicated otherwise. P values < 0.05 were considered statistically significant. In addition, in order to evaluate visually whether the suppressive effect of eradication therapy on gastric cancer tends to change with time, we plotted the RD on the y-axis against the mean follow-up period for the interventional and control groups of each study on the x-axis, and fit a simple linear regression line based on the least squares method.
Results
Search results
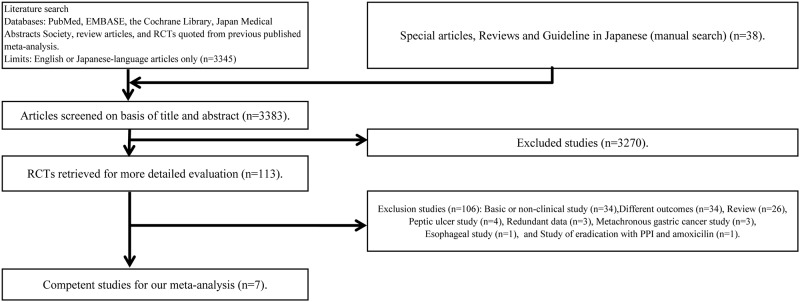
We screened 3383 studies through database searches and by reading through publications. Of these, we excluded 3270 studies and evaluated 113 in detail, and were ultimately left with seven studies (Fig 1) [43–49]. An RCT by Wong et al. compared four groups including a placebo group [48]. Of these, the two groups that met the inclusion criteria and were thus extracted for this study included one for which treatment intervention was a typical eradication therapy, and placebo administration for the other. Of the 106 studies excluded, we confirmed three article sets that involved multiple articles for a single study, in which the subjects and study background were identical but the study period differed. The studies by Zhou et al.[49], Mera et al.[45], and Ma et al.[47] were the long-term versions of those by Leung et al.[7], Correa et al.[50], and You et al. [51], respectively. Prior to their final report [49], Zhou et al. had also published a summarized version [52]. In accordance with the methodology described above, we excluded short-term studies with the same background and ultimately included their long-term counterparts (each published after the short-term study). Although the study by Miehke et al.[44] was not included in previous four meta-analyses [6, 9–11], we included it here as it met our inclusion criteria. None of the RCTs in the Japan Medical Abstracts Society or the three gastrointestinal medical journals satisfied the inclusion criteria. The article by Saito et al. was an abstract published as a poster at an academic conference (Digestive Disease Week, DDW 2005) [46]. Because the subsequent clinical study was not presented as an article, only the conference abstract was used. The research results were first published in Japanese; we did not find any article that later reported the same results in English.
Fig 1. Flow of randomized controlled trials through the process of retrieval and inclusion in the meta-analysis comparing eradication treatment for Helicobacter pylori infection.
RCT, Randomized controlled trial.
Study characteristics
Characteristics of all seven studies included in the final analysis [43–49] are summarized in Table 2. An intention-to-treat (ITT) analysis had been carried out in all of the studies, which were all published in English. Five of the seven studies were multicenter studies [43, 44, 46, 47, 49]. Subjects were normal healthy individuals in four studies [44–46, 48] and unspecified in three [43, 47, 49]; none of the studies clearly stated that the subjects were patients. Upper abdominal symptoms during the study were absent in three studies [44–46] and unspecified in four [43, 47–49]. The countries participating in the studies were China in four studies [44, 47–49]; Japan in one [46]; Columbia in one [45], and Austria, Czech Republic, and Germany in a jointly reported study [43]. Treatment details were as follows:
Table 2. Characteristics of primary trials.
| Author | Reference | Year | Format | Setting | Participants' background | Country | Treatment of | Method of assessing Helicobacter pylori infection | Outcome | Mean age of | Participants in the initial allocation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Participants | Intervention group | Control group | Duration (days) | Primary | Secondary | Intervention group | Control group | Intervention group | Control group | |||||||
| Miehlke S | 43 | 2001 | Full | Multicenters | University and general hospitals | Unclear | Austria, Czech Republic, and Germany | Omeprazole, 20mg; clarithromycin, 500mg; and amoxicillin, 1000mg, all twice daily | PPI | 7 | Histology 13C-urea breath test | Incidence of gastric cancer | None | Unclear | Unclear | 86 | 81 |
| Wong BCY | 44 | 2004 | Full | Multicenters | Public health bureau | Good health | China | Omeprazole, 20mg; metronidazole, 400mg; and amoxicillin, 750mg, all twice daily | Placebo | 14 | 13C-urea breath test | Incidence of gastric cancer | None | 42.0 | 42.0 | 817 | 813 |
| Mera R | 45 | 2005 | Full | Single | Public health bureau | Good health | Colombia | Amoxicillin; metronidazole; and bismuth subsalicylate | Supplement | 14 | Histology | Mucosal healing | Incidence of gastric cancer | Unclear | Unclear | 394 | 401 |
| Saito D | 46 | 2005 | Abstract | Multicenters | University and general hospitals | Good health | Japan | Lansoprazole, 30mg; clarithromycin, 200mg; and amoxicillin, 750mg, all twice daily | None | 7 | Unclear | Mucosal healing | Incidence of gastric cancer | Unclear | Unclear | 379 | 313 |
| Ma JL | 47 | 2012 | Full | Multicenters | Unclear | Unclear | China | Lansoprazole, 30mg; clarithromycin, 200mg; and amoxicillin, 750mg, all twice daily | Placebo | 7 | 13C-urea breath test Serum antibody | Incidence of gastric cancer | None | 47.0 | 47.0 | 1130 | 1128 |
| Wong BCY | 48 | 2012 | Full | Multicenters | University | Unclear | China | Omeprazole, 20mg; clarithromycin, 500mg; and amoxicillin, 1000mg, all twice daily | Placebo | 7 | 13C-urea breath test | Mucosal healing | Incidence of gastric cancer | 53.0 | 52.9 | 255 | 258 |
| Zhou LY | 49 | 2014 | Full | Single | Public health bureau | Good health | China | Omeprazole, 20mg; clarithromycin, 500mg; and amoxicillin, 1000mg, all twice daily | Placebo | 7 | 13C-urea breath test | Mucosal healing | Incidence of gastric cancer | 62.1 | 62.2 | 276 | 276 |
PPI, Proton pump inhibitor
Treatment in the intervention group was PCA (PPI + clarithromycin + amoxicillin) in five studies [43, 46–49], BMA (bismuth + metronidazole + amoxicillin) in one [45], and PMA (PPI + metronidazole + amoxicillin) in one [44]. Treatment in the control group was placebo in four studies [44, 47–49], a supplement in one [45], no treatment in one [46], and PPI in one [43]. With the exception of one study [45], none of the studies had performed secondary H. pylori eradication therapy. The method for assessing H. pylori infection was the 13C-Urea breath test in five studies [43, 44, 47–49], histology in two [43, 45], serum antibodies in one [49], and unspecified in one [47]. The number of times the effectiveness of H. pylori eradication was confirmed was only once in five studies [43–45, 47, 49], four times in one (by a breath test at the time of each endoscopy) [48], and unspecified in one [46].
In terms of outcome evaluation, gastric cancer occurrence had been designated as the primary outcome measure in three studies [43, 44, 49], while a secondary outcome measure was specified in four [45–48]. In an upper gastrointestinal endoscopic examination following eradication therapy, one study [45] found significant improvement of the gastric mucosa whereas three found no significant improvement [46–48]; the other three did not assess this parameter [43, 44, 49].
The total numbers of subjects for the interventional and control groups were 3337 and 3270, respectively, with a mean age of 51.0 years for both groups. Mean follow-up periods for the interventional and control groups were 7.8 and 6.7 years, respectively; the rationale for the follow-up period was not indicated in any study. Two studies [44, 48] reported the time to cancer onset following eradication therapy, and indicated mean times of 3.9 and 2.3 years for the interventional and control groups, respectively.
Histological assessment, using the updated Sydney System score before and after eradication therapy, was not possible for any of the studies. Endoscopy was performed annually in one study [43] or following a fixed non-annual schedule in four studies [44, 45, 47, 48]; the schedule was unspecified in two studies [46, 49].
We sent e-mail inquiries to six of the eight corresponding authors [43–45, 47–49] whose addresses were listed, asking if multiple articles with redundant data had been published. E-mails sent to the listed addresses for two of these six authors did not reach their destinations [45, 49]. None of the remaining four authors replied within the first week; therefore, we sent a reminder to all of these authors [43, 44, 47, 49]. Because we received no replies even after another week, we conducted no further follow-ups.
Study quality
We first evaluated the quality of the seven RCTs in Table 3. The median Jadad score was 3.0 (range: 1–5). The primary outcome measure was described in the methodology of every study. Sample size had been specified in advance in three studies [44, 47, 49]. The funding source was public in four studies [44, 45, 47, 49] and unspecified in three [43, 46, 48]. None of the studies mentioned whether there were any conflicts of interest. In addition, although one study indicated that drugs were actually provided, six lacked such description [43–48].
Table 3. Evidence quality of each RCT used.
| Author | Reference | Description of | Description of grant | Limitations (Risk of bias) for each RCT | Jadad score | Quality of study | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main outcome in methods | Basis of sample size calculation in methods | Fund | Fund was supplied by sponsor company | Medicine was supplied by sponsor company | Allocation concealment | Adequate sequence generation | Blinding | Incomplete outcome data addressed | Free of elective outcome reporting | Free of other bias | Randomization | Appropriateness of randomization | Double blind | Appropriateness of double blind | Dropout | Sum | |||
| Miehlke S | 43 | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Unclear | 1 | 0 | 1 | 0 | 1 | 3 | High |
| Wong BCY | 44 | Yes | Yes | Public | No | No | Yes | Yes | Yes | Yes | Yes | Unclear | 1 | 1 | 1 | 1 | 1 | 5 | High |
| Mera R | 45 | Yes | No | Public | No | No | No | Yes | No | Unclear | Yes | Unclear | 1 | 0 | 0 | 0 | 1 | 2 | Low |
| Saito D | 46 | Yes | No | No | No | No | No | Yes | No | Unclear | Yes | Unclear | 1 | 0 | 0 | 0 | 0 | 1 | Low |
| Ma JL | 47 | Yes | Yes | Public | No | Yes | No | Yes | No | Unclear | Yes | Unclear | 1 | 0 | 1 | 0 | 0 | 3 | High |
| Wong BCY | 48 | Yes | Yes | Public | No | No | Yes | Yes | Yes | Unclear | Yes | Unclear | 1 | 1 | 1 | 0 | 1 | 4 | High |
| Zhou LY | 49 | Yes | No | No | No | No | No | Yes | No | Unclear | Yes | Unclear | 1 | 0 | 1 | 0 | 1 | 3 | High |
RCT, Randomized controlled trial
Regarding the risk of bias for each RCT, three studies mentioned allocation concealment [43, 44, 47], all noted adequate sequence generation, and three mentioned blinding [43, 44, 49]. Only two studies noted inadequate outcome data [43, 44], and free of elective outcome reporting was mentioned in all studies. None of the studies commented on whether they were free of other biases. Consequently, the quality of each study was moderate, as was the quality of the body of evidence in general.
Suppressive effect of H. pylori eradication therapy on primary gastric cancer
Overall, eradication therapy of H. pylori infection significantly reduced the risk on primary gastric cancer (pooled risk ratio [RR], 0.67; 95% Confidence Interval [CI], 0.48 to 0.95 with low heterogeneity (I2 = 0%) in Table 4 and Fig 2. A subgroup analysis, the pooled RR was significantly shown for RCTs of high quality, those within Asia, those in which the control group was given a placebo or no treatment, those that targeted subjects with a mean age between 41 and 50 years, and even those in which the mean study period exceeded 10 years.
Table 4. Effectiveness of Helicobacter pylori eradication therapy for gastric cancer prevention.
| Outcomes | Reference | No. of studies | Pooled risk ratio | Heterogeneity | Pooled risk difference | Heterogeneity | Statistical method by effect model | NNT | Quality of a body of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | 95%CI | I2 value (%) | Value | 95%CI | I2 value (%) | Value | 95%CI | ||||||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| Overall | 43–49 | 7 | 0.67 | 0.48 | 0.95 | 0 | -0.00 | -0.01 | 0.00 | 33 | D-L | 125.5 | 70.0 | 800.9 | Moderate |
| High quality RCTs | 43, 44, 47–49 | 5 | 0.65 | 0.45 | 0.93 | 0 | -0.01 | -0.02 | 0.00 | 50 | D-L | 101.7 | 57.4 | 604.0 | |
| RCT in Japan | 46 | 1 | 0.55 | 0.09 | 3.27 | Uncalculatable | -0.00 | -0.02 | 0.01 | Uncalculatable | - | 232.1 | 79.8 | ∞ | |
| Studies within Asia | 44, 46–49 | 5 | 0.64 | 0.45 | 0.92 | 0 | -0.01 | -0.01 | 0.00 | 47 | D-L | 102.7 | 59.6 | 463.2 | |
| Studies outside of Asia | 43, 45 | 2 | 1.27 | 0.34 | 4.70 | Uncalculatable | 0.00 | -0.01 | 0.02 | 0 | M-H | 472.2 | 85.7 | ∞ | |
| Annual healthy screening or good health | 44–46, 49 | 4 | 0.63 | 0.34 | 1.17 | 0 | -0.01 | -0.01 | 0.00 | 0 | M-H | 185.8 | 88.4 | ∞ | |
| Giving placebo or no treatment to comparison group | 44, 46–49 | 5 | 0.64 | 0.45 | 0.92 | 0 | -0.01 | -0.01 | 0.00 | 47 | D-L | 102.7 | 59.6 | 463.2 | |
| Cases of gastric mucosal improvement after eradication | 45 | 1 | 1.27 | 0.34 | 4.70 | Uncalculatable | 0.00 | -0.01 | 0.02 | Uncalculatable | - | 368.3 | 70.1 | ∞ | |
| Patients with preneoplastic lesions | 44, 47, 48 | 3 | 0.69 | 0.47 | 1.00 | 0 | -0.00 | -0.02 | 0.01 | 68 | D-L | 77.7 | 38.0 | ∞ | |
| Patients without preneoplastic lesions | 43, 45, 46, 49 | 4 | 0.62 | 0.27 | 1.43 | 6 | -0.00 | -0.01 | 0.00 | 0 | M-H | 155.4 | 90.1 | ∞ | |
| Participants' mean age, 41 to 50 | 44, 47 | 2 | 0.65 | 0.44 | 0.96 | 0 | -0.01 | -0.02 | 0.00 | 46 | D-L | 87.7 | 48.1 | 794.6 | |
| Age 51 to 60 | 48 | 1 | 3.04 | 0.32 | 29.0 | Uncalculatable | 0.01 | -0.01 | 0.02 | Uncalculatable | - | 126.8 | 70.1 | ∞ | |
| Gastric cancer, gastric type | 43, 48 | 2 | 3.04 | 0.32 | 29.0 | Uncalculatable | 0.01 | -0.01 | 0.02 | 0 | M-H | 535.0 | Uncalculatable | ||
| Gastric cancer, intestinal type | 43, 48 | 2 | 3.04 | 0.32 | 29.0 | Uncalculatable | 0.01 | -0.01 | 0.02 | 0 | M-H | 535.0 | Uncalculatable | ||
| Gastric cancer, cardiac type | 43 | 1 | Uncalculatable | Uncalculatable | 0.00 | -0.02 | 0.02 | Uncalculatable | - | Uncalculatable | |||||
| Gastric cancer, non cardiac type | 43 | 1 | Uncalculatable | Uncalculatable | 0.00 | -0.02 | 0.02 | Uncalculatable | - | Uncalculatable | |||||
| Annual follow-up of endoscopic examination | 43 | 1 | Uncalculatable | Uncalculatable | 0.00 | -0.02 | 0.02 | Uncalculatable | - | Uncalculatable | |||||
| Scheduled follow-up except annual endoscopic examination | 44, 45, 48, 49 | 4 | 0.74 | 0.40 | 1.38 | 19 | -0.00 | -0.01 | 0.01 | 37 | D-L | 294.2 | 102.2 | ∞ | |
| Research duration (mean), shorter than 5 years | 43, 46 | 2 | 0.55 | 0.09 | 3.27 | Uncalculatable | -0.00 | -0.01 | 0.01 | 0 | M-H | 301.8 | 100.7 | ∞ | |
| 5 to 10 years | 44, 48 | 2 | 0.98 | 0.25 | 3.89 | 37 | 0.00 | -0.01 | 0.01 | 48 | D-L | 533.0 | 105.7 | ∞ | |
| Longer than 10 years | 45, 47, 49 | 3 | 0.65 | 0.44 | 0.96 | 4 | -0.01 | -0.02 | 0.01 | 55 | D-L | 82.5 | 45.0 | 833.3 | |
| Eradication therapy, PCA | 43, 46–49 | 5 | 0.65 | 0.44 | 0.95 | 0 | -0.01 | -0.02 | 0.01 | 53 | D-L | 86.9 | 49.4 | 489.3 | |
| PMA | 44 | 1 | 0.63 | 0.25 | 1.63 | Uncalculatable | -0.00 | -0.02 | 0.01 | Uncalculatable | - | 201.5 | 76.1 | ∞ | |
| BMA | 45 | 1 | 1.27 | 0.34 | 4.70 | Uncalculatable | 0.00 | -0.01 | 0.02 | Uncalculatable | - | 368.3 | 70.1 | ∞ | |
CI, confidence intervals; NNT, Number needed to treat; RCT, Randomized controlled trial; M-H, Mantel-Haenszel; D-L, DerSimonian-Laird
PCA, Proton pump inhibitor, clarithromycin, and amoxicillin; PMA, Proton pump inhibitor, metronidazole, and amoxicillin; BMA, bismuth, metronidazole, and amoxicillin
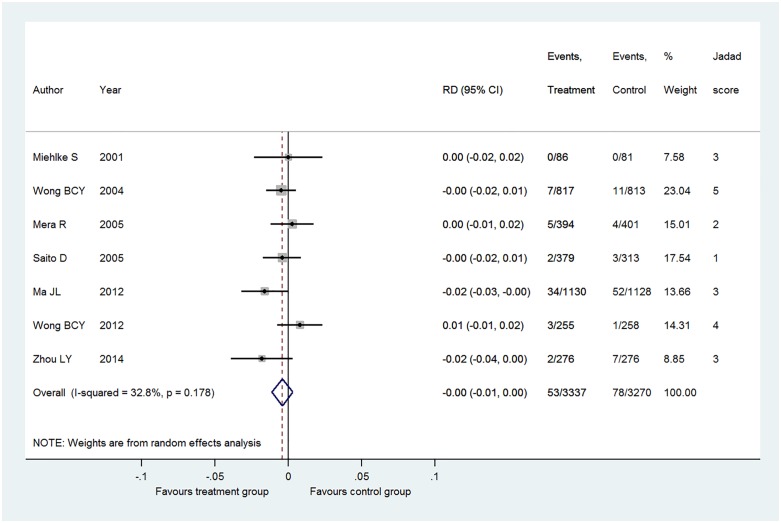
Fig 2. Pooled risk difference (RD) in gastric cancer occurrence in patients with Helicobacter pylori infection.
I2 value indicates heterogeneity of 33%. n = case of gastric cancer. N = group size.
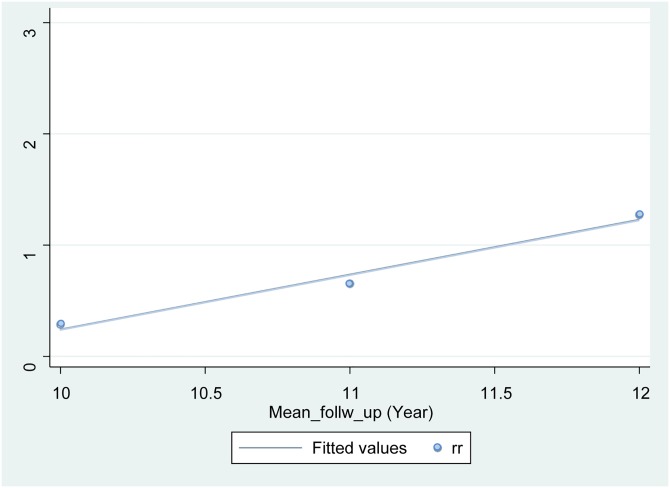
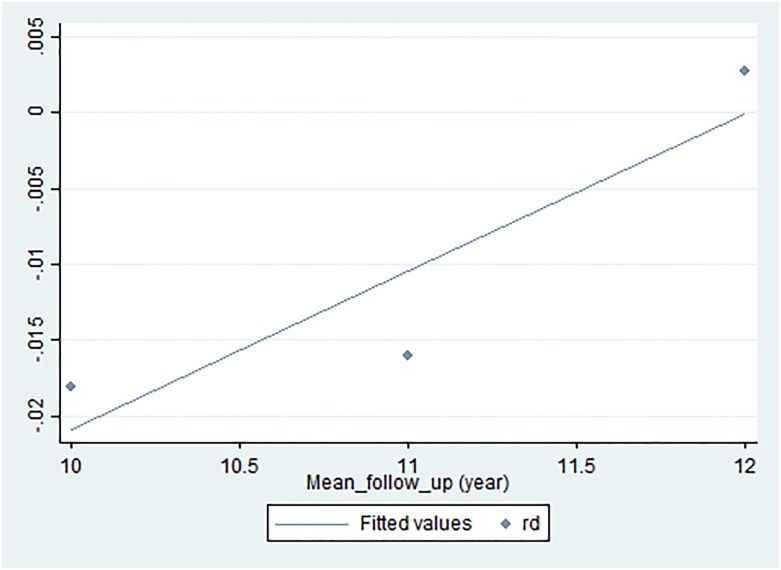
Overall, eradication therapy of H. pylori infection did not significantly reduce the risk on primary gastric cancer (pooled difference [RD], -0.00; 95% Confidence Interval [CI], -0.01 to 0.00 with low heterogeneity (I2 = 33%) and NNT = 125.5 [95% CI: 70.0 to 800.9]). When the analysis was restricted to the five high-quality RCTs, pooled RD was -0.01 [95% CI: -0.02 to 0.00] (I2 = 50%, NNT = 101.7 [95% CI: 57.4 to 604.0]). When analyzing the relationship between RR and follow-up period for the three studies with a mean follow-up period of at least 10 years [45, 48, 49], linear regression yielded a line with a positive slope (y (RR) = 0.4932x (year)– 4.6888) (Fig 3). Besides, when analyzing the relationship between RD and follow-up period for the three studies with a mean follow-up period of at least 10 years [45, 48, 49], linear regression yielded a line with a positive slope (y (RD) = 1.0416x (year)– 12.504) (Fig 4). This suggests that although the risk of cancer onset is higher in the control group than in the intervention group up to a follow-up period of 11.5 to 12 years, the relationship might be reversed beyond this point.
Fig 3. Simple linear regression.
Risk ratio (y-axis) was plotted as a function of the mean follow-up period in the interventional and control groups of each study (x-axis), and a simple linear regression line was fitted using the least squares method. The point at which the risk of cancer occurrence in the interventional group exceeds that in the control group was calculated to be approximately 11.5 years. rr, risk ratio.
Fig 4. Simple linear regression.
Risk difference (y-axis) was plotted as a function of the mean follow-up period in the interventional and control groups of each study (x-axis), and a simple linear regression line was fitted using the least squares method. The point at which the risk of cancer occurrence in the interventional group exceeds that in the control group was calculated to be approximately 12 years. rd, risk difference.
Publication bias
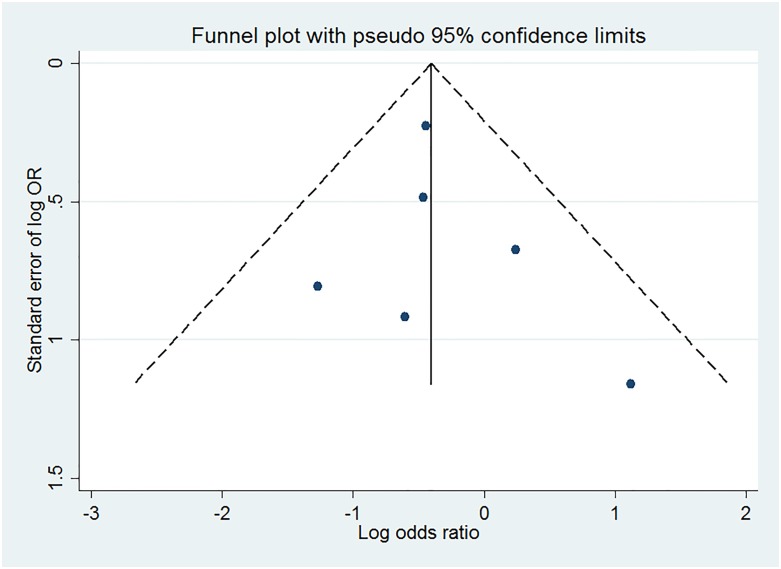
Funnel-plot analysis, Begg’s test, and Egger’s test were performed to evaluate the potential for publication bias in terms of overall RD (Fig 5). The funnel-plot did not show an asymmetric pattern. Neither of the statistical tests revealed significant publication bias (p = 0.348, p = 0.610, respectively).
Fig 5. Publication bias of trials reporting gastric cancer in patients with Helicobacter pylori infection.
OR, Odds Ratio.
Discussion
In this study, we conducted a systematic review of whether H. pylori eradication therapy suppresses the occurrence of primary gastric cancer in patients whose gastric cancer was not diagnosed endoscopically. Three RCTs [43, 45, 48] were added to the meta-analysis by Fuccio et al.[6] in 2009, and one [43] to the meta-analyses by Ford et al.[9, 10] in 2014 and 2015. A meta-analysis of the seven articles ultimately included revealed that, whereas the gastric cancer suppressing effect of eradication therapy was comparable to that of previous research in terms of RR, the effect size in terms of RD, which was not assessed in previous studies, was minor and not statistically significant. Overall NNT was 125, and subgroup analyses were approximately from 100 to 300. Showing these values, we think the effectiveness of eradication of Helicobacter pylori to prevent primary gastric cancer was relatively low. This trend was observed in all subgroup analyses in Table 4.
In this study, the overall pooled RR for the suppression of gastric cancer by H. pylori eradication was 0.67 (95% CI: 0.48 to 0.95), which was comparable to that of previous studies. However, when expressed in terms of RD, the overall pooled RD was -0.00 (95% CI: -0.01 to 0.00), i.e., the effect was slight and not statistically significant. It is common that "Relative Risk obtained from the same data looks larger than Risk Difference". Apart from whether it is one case symbolically seen in the present research question and whether it is deliberate or not intentional, in the previous study both RCT and meta-analysis were only indicators of risk ratio. The magnitude of the effect such as risk ratio 0.6–0.7 was emphasized, and the tone of the discussion was to justify the aggressive intervention. Although we used the same data to analyze pooled risk ratio and risk difference, pooled risk ratio was statistically significant but pooled risk difference was not. When the event-incidence was rare with meta-analysis, in another study, pooled risk ratio was statistically significant but pooled risk difference was not like our study [53]. We thought that the association between pooled risk ratio and risk difference showed discrepancy because we used data of rare events. In fact, Lane, et al showed that this discrepancy occurred when the methods of statistical tests in the meta-analysis were the differences and outcome incidence of the original data used in randomized controlled trials was rare. Main purpose of our study is to facilitate careful discussion on the expected magnitude of the effect of sterilization by H. pylori eradication to prevent primary gastric cancer comparing two risk indices that have been rarely mentioned before. We think that this phenomenon can be called a new outcome- reporting bias in risk communication. Many epidemiological findings, including those from clinical trials, are expressed in terms of RR; however, it has been repeatedly pointed out that compared with absolute risk, this index overestimates the association. [21]. For example, in a clinical trial by Lipid Research Clinics reported in 1982 [54], a 19% reduction in the risk of ischemic heart disease due to a cholesterol-reducing drug was emphasized. However, this was expressed in terms of relative risk reduction (RRR); the absolute risk reduction (ARR) was 1.6%. A similar report also emphasized an RRR of 31% over an ARR of 2.3% [55]. The RRR used in these studies, although not identical to relative risk, is a ratio-based index; this expression was likely used with the intent to strengthen reader impressions. Given these findings, the FDA proposed the following in a 2011 report when communicating risk: “Provide absolute risks, not just relative risks. Patients are unduly influenced when risk information is presented using a relative risk approach; this can result in suboptimal decisions” [13]. The RR results presented herein do not deny the effectiveness of H. pylori eradication in preventing cancer. Nonetheless, the results for absolute risk (AR) suggest that we should be cautious regarding the effect size and its level of certainty as evidence. Typically, the problem with selective reporting has been outcome reporting bias, in which numerous outcomes are measured and only those variables attaining statistical significance are published [56–58]. Previous studies of H. pylori eradication presented their results only as a ratio index that emphasizes its effect could be pointed out as a new selective reporting issue distinct from prevailing problems.
NNT is a treatment effect index based on ARR. Fuccio et al.[6] or Lee et al. [11] did not show NNT in their report. Although Ford et al.[9] showed NNT separately by country, their interpretation of this in their report was that the treatment would reduce the occurrence of gastric cancer within Asia. Obtaining the NNT for each population is appropriate because this index is influenced by the AR of a disease in a target population. However, a stable NNT cannot be obtained from findings in a single-population study that lacks power. To make evidence-based decisions, it will be necessary to alleviate the large impact that RR has on readers by obtaining NNT from an integrated RD and interpreting the results within the article.
To whom can we recommend H. pylori eradication therapy for suppressing the occurrence of gastric cancer? Eradication therapy is necessary for patients who have just undergone early-stage gastric cancer surgery [59–62] and is well-established for patients who have undergone endoscopic therapy for this type of cancer [5, 63]. This study, which targeted asymptomatic patients infected with H. pylori, predicted the following: first, that the reduction in the risk of gastric cancer by H. pylori eradication therapy would be at least 30% in terms of RR, similar to that of previous findings, but would be slight and statistically insignificant in terms of RD; and second, that after eradication, a suppressive effect on gastric cancer would likely occur in the short term but diminish in the long term due to increased occurrence of cancer from aging (Fig 3). Therefore, those with severe gastric mucosal atrophy or precancerous lesions, which are relatively elderly high-risk groups, tend not to benefit from the treatment effects. In contrast, young individuals without atrophy might be expected to benefit from the effects of eradication therapy in preventing gastric cancer. In the long-term, endoscopy will likely be necessary because cancer begins to occur more frequently with age, even in the intervention group.
This study has several limitations. First, the mean study periods of the RCTs used were short (6–7 years). In a review which cited an article by Graham et al. and the fact that the occurrence of cancer had also been demonstrated in RCTs with long-term follow-up, Tan et al. [64] stated that cohort studies on the same topic should also be referred to in evaluating the gastric cancer suppressing effects of H. pylori eradication therapy. Considering that many of the RCTs used here were completed within a few years, a re-examination including a cohort study with a longer observation period would be meaningful. For observational studies, pre-registration has not become as widespread as it has for RCTs; such studies should also be interpreted cautiously because publication bias is more severe than it is for interventional studies, and because cohort studies, given their high bias risk, may show results that diverge from true results. Second, the eradication effect could vary depending on histopathology, i.e., gastritis, atrophy, intestinal metaplasia, or dysplasia. However, these could not be clearly distinguished in the present study.
There is ABC classification for assessing gastric cancer risk and evaluating the degree of gastric mucosal atrophy objectively and quantitatively [65]. In our study, we couldn’t compare the degree of gastric mucosal atrophy quantitatively. Among youths, a H. pylori carrier without gastric mucosal atrophy could possibly lower the risk of primary gastric cancer [66]. This problem could be addressed by future studies with the development of a standardized method for tissue evaluation.
From the present study, we conclude that the suppressive effect of H. pylori eradication therapy on the occurrence of primary gastric cancer was statistically significant and comparable to that of previous studies in terms of estimated RR. However, in terms of RD, the effect size was minor and not statistically significant. At this point, caution must be exercised when promoting evidence-based eradication measures.
Supporting information
(DOC)
Abbreviations
- AR
Absolute risk
- ARR
Absolute risk reduction
- BMA
bismuth, metronidazole, and amoxicillin
- CI
Confidence interval
- COI
Conflict of interest
- DDW
Digestive Disease Week
- EMBASE
Excerpta Medica database
- EMR
Endoscopic mucosal resection
- ESD
Endoscopic submucosal dissection
- FDA
Food and Drug Administration
- ITT
Intention to treat
- NNT
Number needed to treat
- PCA
Proton pump inhibitor, clarithromycin, and amoxicillin
- PMA
Proton pump inhibitor, metronidazole, and amoxicillin
- PPI
Proton pump inhibitor
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- RD
Risk difference
- RR
Risk ratio
- RRR
Relative risk reduction
Data Availability
Data are available from PubMed, EMBASE, Google scholar, the Cochrane Library, and the Japan Medical Abstracts Society as well as those registered in databases of the Cochrane Central Register of Controlled Trials, metaRegister of Controlled Trials, ClinicalTrials.gov, controlled-trials.com, UMIN-CTR, JMACCT-CTR, and JAPIC-CTI.
Funding Statement
This study was supported by a grant-in-aid from the Japan Primary Care Association in 2015.
References
- 1.International Agency for Research on Cancer. World Health Organization. Infection with Helicobacter pylori In: Schistosomes, liver flukes and Helicobacter pylori. Lyon: IARC, 1994, 177–202. [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–40. . [PubMed] [Google Scholar]
- 3.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol 2005; 100:1037–42. doi: 10.1111/j.1572-0241.2005.41384.x . [DOI] [PubMed] [Google Scholar]
- 4.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol 2007; 42 Suppl 17: 21–7. doi: 10.1007/s00535-006-1924-9 . [DOI] [PubMed] [Google Scholar]
- 5.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008; 372: 392–7. doi: 10.1016/S0140-6736(08)61159-9 . [DOI] [PubMed] [Google Scholar]
- 6.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009; 151: 121–8. . [DOI] [PubMed] [Google Scholar]
- 7.Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004; 53: 1244–9. doi: 10.1136/gut.2003.034629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford AC, Moayyedi P. Redundant data in the meta-analysis on Helicobacter pylori eradication. Ann Intern Med 2009; 151: 513 . [DOI] [PubMed] [Google Scholar]
- 9.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014; 348: g3174 doi: 10.1136/bmj.g3174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev 2015; 7: CD005583 doi: 10.1002/14651858.CD005583.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016; 150: 1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028 . [DOI] [PubMed] [Google Scholar]
- 12.Porta M, ed. A Dictionary of Epidemiology, six edition: Oxford University Press; 2014. [Google Scholar]
- 13.Fagerlin A (2011).”Chapter 7: Quantitative Information”. In: Fischhoff B, Brewer NT, Downs JS (eds.) Communicating Risks and Benefits: An Evidence-Based User's Guide, 57–61. Annapolis: Food and Drug Administration (FDA). http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM268069.pdf
- 14.Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992; 117: 916–21. . [DOI] [PubMed] [Google Scholar]
- 15.Elting LS, Martin CG, Cantor SB, Rubenstein EB. Influence of data display formats on physician investigators' decisions to stop clinical trials: prospective trial with repeated measures. BMJ. 1999; 318: 1527–31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010; 152: 726–32. doi: 10.7326/0003-4819-152-11-201006010-00232 . [DOI] [PubMed] [Google Scholar]
- 17.Nuovo J, Melnikow J, Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA. 2002; 287: 2813–4. . [DOI] [PubMed] [Google Scholar]
- 18.Schechtman E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat—which of these should we use? Value Health. 2002; 5: 431–6. doi: 10.1046/J.1524-4733.2002.55150.x . [DOI] [PubMed] [Google Scholar]
- 19.Citrome L. Relative vs. absolute measures of benefit and risk: what's the difference? Acta Psychiatr Scand. 2010; 121: 94–102. doi: 10.1111/j.1600-0447.2009.01449.x . [DOI] [PubMed] [Google Scholar]
- 20.Nakayama T. Under-reporting of attributable risk and reporting of the risk ratio in epidemiologic literature. Epidemiology. 2000; 11: 366–7. . [DOI] [PubMed] [Google Scholar]
- 21.Nakayama T, Zaman MM, Tanaka H. Reporting of attributable and relative risks, 1966–97. Lancet. 1998; 351: 1179 doi: 10.1016/S0140-6736(05)79123-6 . [DOI] [PubMed] [Google Scholar]
- 22.Gigerenzer G, Wegwarth O, Feufel M. Misleading communication of risk. BMJ. 2010; 341: c4830 doi: 10.1136/bmj.c4830 . [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–9. . [DOI] [PubMed] [Google Scholar]
- 24.JMAS (Japan Medical Abstracts Society, http://login.jamas.or.jp/)
- 25.Cochrane Central Register of Controlled Trials, http://onlinelibrary.wiley.com/cochranelibrary/search?searchRow.searchOptions.searchProducts=clinicalTrialsDoi
- 26.metaRegister of Controlled Trials, http://www.controlled-trials.com/mrct/
- 27.ClinicalTrials.gov, http://clinicaltrials.gov.
- 28.controlled-trials.com, http://www.controlled-trials.com/
- 29.UMIN (University Hospital Medical Information Network in Japan, http://www.umin.ac.jp/ctr/index-j.htm)
- 30.JMACCT (Japan Medical Association Clinical Trial Registry, https://dbcentre3.jmacct.med.or.jp/jmactr/App/JMACTRS01/JMACTRS01.aspx?kbn=4)
- 31.JAPIC (Japan Pharmaceutical Information Center, http://clinicaltrials.jp/user/cte_main.jsp) [DOI] [PubMed]
- 32.Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database Syst Rev 2011; 11: MR000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–81. . [DOI] [PubMed] [Google Scholar]
- 34.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. . [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Tating the quality of evidence-study limitations (risk of bias). J Clin Epidemiol 2011; 64: 407–415. doi: 10.1016/j.jclinepi.2010.07.017 . [DOI] [PubMed] [Google Scholar]
- 36.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41: 55–68. . [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring incomsistency in meta-analysis. BMJ 2003; 327: 557–560. doi: 10.1136/bmj.327.7414.557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. . [DOI] [PubMed] [Google Scholar]
- 39.Light RJ, Pilemer DB. Summing Up: The Science of Reviewing Research. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 40.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. . [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 43.Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, et al. Helicobacter pylori and gastric cancer:current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study). World J Gastroenterol 2001; 7: 243–7. doi: 10.3748/wjg.v7.i2.243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004; 291: 187–94. doi: 10.1001/jama.291.2.187 . [DOI] [PubMed] [Google Scholar]
- 45.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005; 54: 1536–40. doi: 10.1136/gut.2005.072009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito D, Boku N, Fujioka T. Impact of H. Pylori Eradication on Gastric Prevention: Endoscopic Results of the Japanese Intervention Trial (JITHP-Study). a Randomized Multi-Center Trial. Gastroenterol 2005; 128(4 Suppl 2): A4. [Google Scholar]
- 47.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012; 104: 488–92. doi: 10.1093/jnci/djs003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012; 61: 812–8. doi: 10.1136/gutjnl-2011-300154 . [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J 2014; 127: 1454–8. . [PubMed] [Google Scholar]
- 50.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000; 92: 1881–8. . [DOI] [PubMed] [Google Scholar]
- 51.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst 2006; 98: 974–83. doi: 10.1093/jnci/djj264 . [DOI] [PubMed] [Google Scholar]
- 52.Zhou LY. Ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China. Gastroenterol 2008; 134: A233. [Google Scholar]
- 53.Lane PW. Meta-analysis of incidence of rare events. Stat Methods Med Res. 2013; 22: 117–32. doi: 10.1177/0962280211432218 . [DOI] [PubMed] [Google Scholar]
- 54.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984; 251: 351–64. . [DOI] [PubMed] [Google Scholar]
- 55.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–7. doi: 10.1056/NEJM199511163332001 . [DOI] [PubMed] [Google Scholar]
- 56.Raghav KP, Mahajan S, Yao JC, Hobbs BP, Berry DA, Pentz RD, et al. From Protocols to Publications: A Study in Selective Reporting of Outcomes in Randomized Trials in Oncology. J Clin Oncol 2015; 33: 3583–90. doi: 10.1200/JCO.2015.62.4148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Bogert CA, Souverein PC, Brekelmans CT, Janssen SW, van Hunnik M, Koëter GH, et al. Occurrence and determinants of selective reporting of clinical drug trials: design of an inception cohort study. BMJ Open 2015; 5: e007827 doi: 10.1136/bmjopen-2015-007827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dwan K, Altman DG, Clarke M, Gamble C, Higgins JP, Sterne JA, et al. Evidence for the selective reporting of analyses and discrepancies in clinical trials: a systematic review of cohort studies of clinical trials. PLoS Med 2014; 11: e1001666 doi: 10.1371/journal.pmed.1001666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS; Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. [Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea]. Korean J Gastroenterol 2009; 54: 269–78. . [DOI] [PubMed] [Google Scholar]
- 60.Asaka M. [Guidelines in the management of H. pylori infection in Japan—2009 version]. Nihon Rinsho 2009; 67: 2227–32. . [PubMed] [Google Scholar]
- 61.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, et al. Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009; 24: 1587–600. doi: 10.1111/j.1440-1746.2009.05982.x . [DOI] [PubMed] [Google Scholar]
- 62.Hu FL, Hu PJ, Liu WZ, De Wang J, Lv NH, Xiao SD, et al. Third Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis 2008; 9: 178–84. . [DOI] [PubMed] [Google Scholar]
- 63.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56: 772–81. doi: 10.1136/gut.2006.101634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan VP, Wong BC. Gastric cancer chemoprevention: the current evidence. Gastroenterol Clin North Am 2013; 42: 299–316. doi: 10.1016/j.gtc.2013.02.001 . [DOI] [PubMed] [Google Scholar]
- 65.Ichinose M, Yahagi N, Oka M, Ikeda H, Miki K, Omata M. Screening for gastric cancer in Japan In: Wu GY, Aziz K, eds. Cancer screening for common malignancies. Totowa, New Jersey: Humana Press, 200187–102. [Google Scholar]
- 66.Asaka M, Kato M, Graham DY. Strategy for eliminating gastric cancer in Japan. Helicobacter 2010; 15: 486–90. doi: 10.1111/j.1523-5378.2010.00799.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
Data are available from PubMed, EMBASE, Google scholar, the Cochrane Library, and the Japan Medical Abstracts Society as well as those registered in databases of the Cochrane Central Register of Controlled Trials, metaRegister of Controlled Trials, ClinicalTrials.gov, controlled-trials.com, UMIN-CTR, JMACCT-CTR, and JAPIC-CTI.