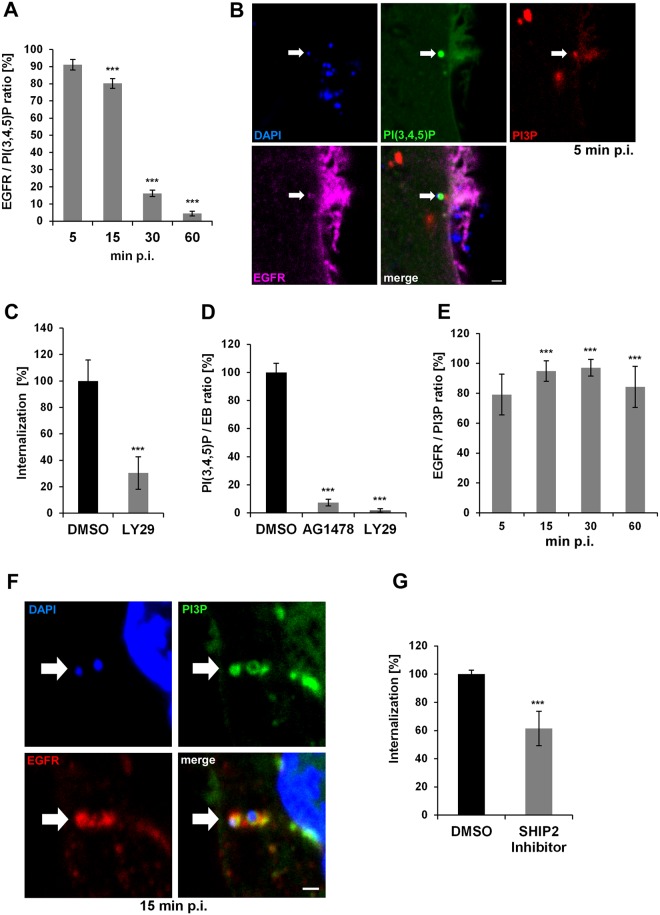
Fig 1. Entry of C. pneumoniae EBs is PI3K dependent.
(A) Quantification of colocalization of PI(3,4,5)P and EGFR in cells transiently transfected with Btk-PH-GFP. Cells were infected at MOI 5, fixed with PFA at the indicated time points (5–60 min), and stained for endogenous EGFR using anti-EGFR, anti-rabbit Alexa594 and DAPI. Confocal images of 30 individual cells were used to analyze colocalization (n = 3). (B) The arrow marks a bacterial entry site at 5 min p.i. visualized by confocal imaging. The bacterial DNA stained with DAPI colocalizes with the “ring-like” signal of PI(3,4,5)P detected with Btk-PH-GFP. Furthermore, the entry site is marked by PI3P detected with mCherry-2xFYVE and the endogenous EGFR surrounding the internalized EB. (C) Quantification of C. pneumoniae internalization in cells pretreated with LY29 (50 μmol) for 2 h prior to infection. At 2 hpi internalization was measured by comparison numbers of internal and external EBs in 30 imaged cells (n = 4). (D) Quantification of EBs (stained with DAPI) colocalizing with PI(3,4,5)P (labeled by Btk-PH-GFP) at 5 min p.i. in cells pretreated with AG1478 (2 μmol) or LY29 (50 μmol) for 2 h (n = 3). (E) Quantification of colocalization of PI3P and EGFR in infected cells expressing GFP-2xFYVE. EGFR was stained as before (n = 3). (F) Colocalization of PI3P, EGFR and internalized EBs at 15 min p.i. by expression of GFP-2xFYVE and detection of EGFR. (G) Quantification of internalization in cells pretreated for 2 h with the SHIP2 inhibitor AS1949490 (10 μmol). *** P value ≤0.001. Bar 1 μm.