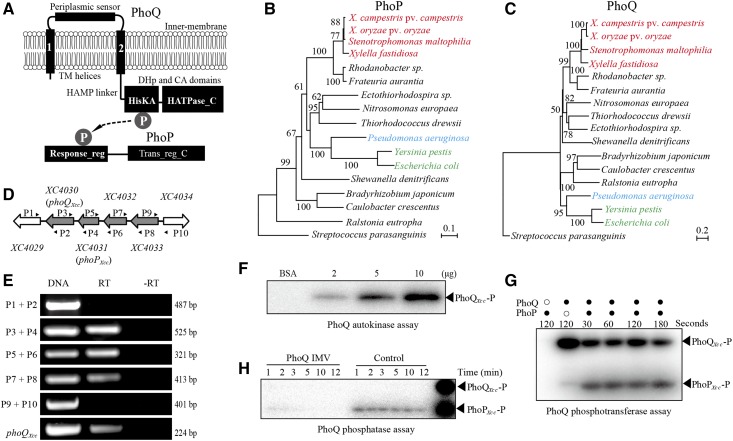
Figure 1.
Phylogeny and signal transduction of the two-component signaling system PhoP-PhoQ. (A) Schematic view of the PhoP and PhoQ secondary structures and cellular locations. TM: transmembrane; protein domain names are indicated according to the pfam database. HisKA (PF00512); HAMP (PF00672); HPt (PF01627); HATPase_c (PF02518); Response_Reg (PF00072); and Trans_reg_C (PF00486). “P” in gray circles represents the phosphoryl group that is transferred from PhoQ to PhoP. (B and C) Phylogenetic relationships of PhoP and PhoQ, respectively. Orthologous protein sequences from representative species of α-, β- and γ-Proteobacteria were used to construct the trees by the Neighbor-Joining method. The scale bar indicates the nucleotide substitutions per site. Orthologous PhoP and PhoQ from the gram-positive bacterium Streptococcus parasanguinis were used as outgroups. Phylogenetic trees were evaluated by bootstrapping (500 duplicates). Bacteria belonging to Xanthomonadaceae, Pseudomonadaceae, and Enterobacteriaceae are listed in red, blue, and green, respectively. (D) Schematic view of the phoPXcc-phoQXcc operon in X. campestris pv. campestris strain 8004. The large arrows represent the location and transcriptional direction of genes; the small black arrows (P1–P10) indicate the primers used in the RT-PCR reactions. (E) Verification of the structure of the phoPXcc-phoQXcc operon by RT-PCR. DNA: positive control using X. campestris pv. campestris 8004 DNA as the PCR template. −RT: no reverse transcriptase negative control. RT: reverse transcriptase added during cDNA synthesis. Bacterial total RNA was used as the template for cDNA synthesis. The experiments were repeated three independent times and a representative is shown. (F) Full-length PhoQXcc has autokinase activity. PhoQXcc-P represents phosphorylated PhoQXcc. Inverted membrane vesicles (IMVs) of PhoQXcc (2–10 µg) were co-incubated with [γ-32P]ATP for 20 min. (G) Full-length PhoQXcc phosphorylates PhoPXcc. PhoQXcc IMVs were autophosphorylated as in (D), and 10 μM of PhoPXcc was then added to the reaction mixtures. Aliquots were taken at the indicated times. Each sample contained 10 μg of PhoQXcc IMVs. (H) PhoQXcc has phosphatase activity toward PhoPXcc. PhoQXcc IMVs were autophosphorylated, and the phosphoryl groups were transferred to PhoPXcc. The IMVs were removed by centrifugation, and the free [γ-32P]ATP was removed by ultrafiltration. The reaction mixture was equally separated into two parts. Unphosphorylated PhoQXcc IMVs and ADP (1 mM) were added to one of the mixtures. Phosphorylated PhoPXcc and PhoQXcc were used as the indicators (the last lane). In (F–H), the reactions were stopped by adding 5 μl of 5 × SDS-PAGE loading buffer. The experiments were repeated three times, and a representative is shown.