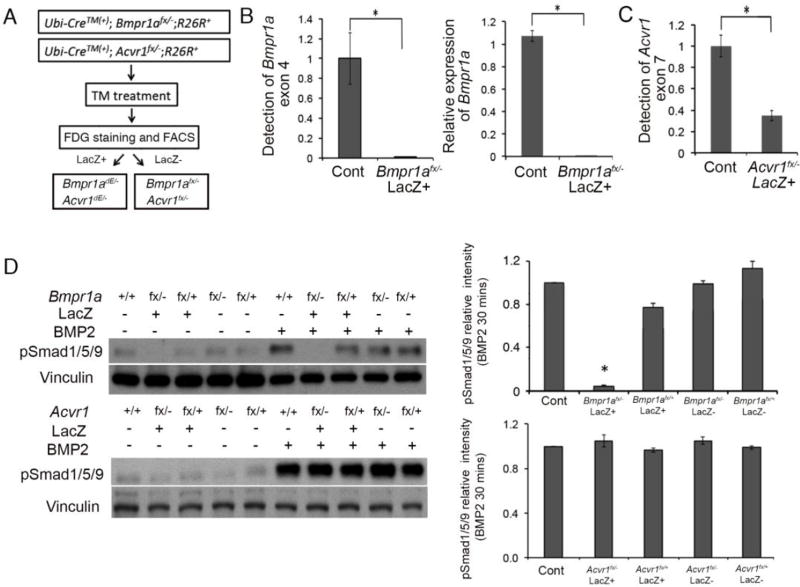
Fig. 6.
Bmpr1a, but not Acvr1 played important role in activating BMP signaling in pre-osteoblast. (A) Workflow of collecting Bmpr1adE/− or Acvr1dE/− cells. Pre-osteoblasts isolated from calvaria of Ubi-CreERT2;Bmpr1afx/−, and Ubi-CreERT2; Acvr1fx/−, then treated with tamoxifen for 6 days. Cells were stained with FDG to separate lacZ(+) and lacZ(−) fractions. Cre-recombined homozygous null cells (Bmpr1adE/− and Acvr1dE/−) should be enriched in the lacZ(+) fractions. (B) Deletion of Bmpr1a exon 4 measured by genomic qPCR and expression levels of Bmpr1a measured by qRT-PCR. (C) Schemes of expected AcvrI wt and fx, Acvr1 null and Acvr1 dE exon structures and resulted sizes of PCR products. RT-PCR for a series samples indicated successful deletion of Acvr1 in cells after enrichment of LacZ expressing cells. LacZ in figure indicated sorted populations. To get Acvr1−/− DNA and RNA, intercross of Acvr1+/− mice was set up and embryos were isolated at E7.5 because of their lethality. (C right) Deletion of Acvr1 exon 7 measured by genomic qPCR. (D) Levels of Phospho-Smad1/5/9 (pSmad1/5/9) were examined in different cells with Bmpr1a mutant and Acvr1 mutant cells. Western blot results were quantified by densitometry (Image J). *, p<0.05.