Figure 7.
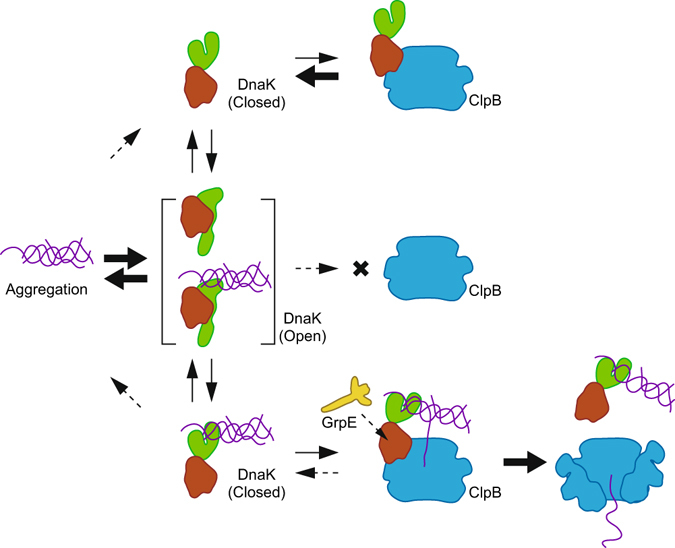
Models for the interactions between chaperones and protein aggregations. DnaK cycles conformational changes between open and closed forms. Although the open form can readily bind and release aggregated proteins, the closed form does not. The closed form of DnaK can bind ClpB and activate it; however, owing to the low affinity, the complex readily dissociates. However, if the bound DnaK holds an aggregation, the ClpB can start threading it. During the threading, the DnaK is tethered by the aggregation to the ClpB and the interaction between NBD and MD of these chaperones is maintained. Consequently, the NBD-MD interaction reduces the approach of GrpE to the DnaK molecule; therefore, the DnaK would maintain the closed form. Thus, the trigonal complex consisting of DnaK, ClpB, and protein aggregation is stabilized. The dissociation of this complex would be effected by accomplishment of the threading and/or collapse of the ClpB ring.
