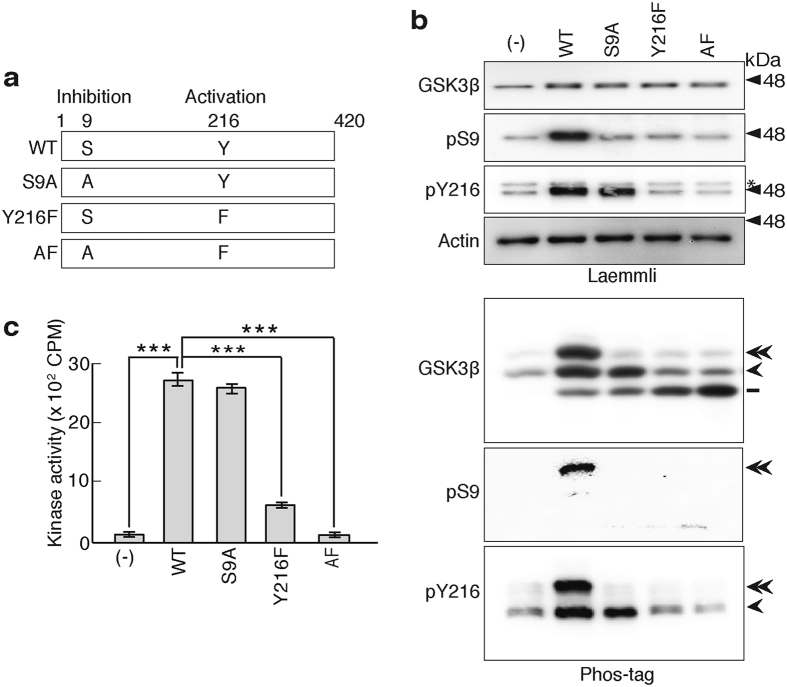
Figure 1.
Separation of the three different phosphoisotypes of GSK3β using Phos-tag SDS-PAGE. (a) Schematic representation of GSK3β and its mutants at the Ser9 and Tyr216 phosphorylation sites. Ser9, whose phosphorylation inhibits the kinase activity, was mutated to Ala (S9A) and Tyr216, whose phosphorylation is required for the activity, was mutated to Phe (Y216F). GSK3β with a double mutation is indicated by AF. (b) Separation of the three GSK3β phosphoisotypes on Phos-tag SDS-PAGE. GSK3β (WT) and its mutants at Ser9 (S9A), Tyr216 (Y216F), or both Ser9 and Tyr216 (AF) were expressed in CHO-K1 cells and subjected to Laemmli’s and Phos-tag SDS-PAGE, followed by immunoblotting with anti-GSK3β, anti-phospho-Ser9 (pS9) and anti-phospho-Tyr216 (pY216) antibodies, as indicated. The left lane shows the control, untransfected cells (-). An asterisk in pY216 blot (third panel) indicates GSK3α. A molecular weight marker of 48 kDa is indicated at the right side of the blots. Actin was used as the loading control in Laemmli’s SDS-PAGE. The amounts of exogenous GSK3β on Phos-tag SDS-PAGE were adjusted prior by immunoblotting with anti-GSK3β after Laemmli’s SDS-PAGE. The phosphorylation states of the three bands of GSK3β on Phos-tag SDS-PAGE are indicated on the right side of the blot; the double arrowhead indicates GSK3β that is phosphorylated at both Ser9 and Tyr216, the arrowhead indicates GSK3β that is phosphorylated at Tyr216, and the bar indicates nonphosphorylated GSK3β. (c) Kinase activity of GSK3β and its mutants. The kinase activity was measured with immunoprecipitated GSK3β and its mutants using a GSK3β substrate peptide and [γ-32P]ATP (means ± s.e.m. n = 3, the data from one of two independent experiments, ***p < 0.001, one-way ANOVA). Uncropped immunoblots of GSK3β, pS9 and pY216 after Laemmli’s and Phos-tag SDS-PAGE are provided in Supplementary Fig. 5.