Figure 1.
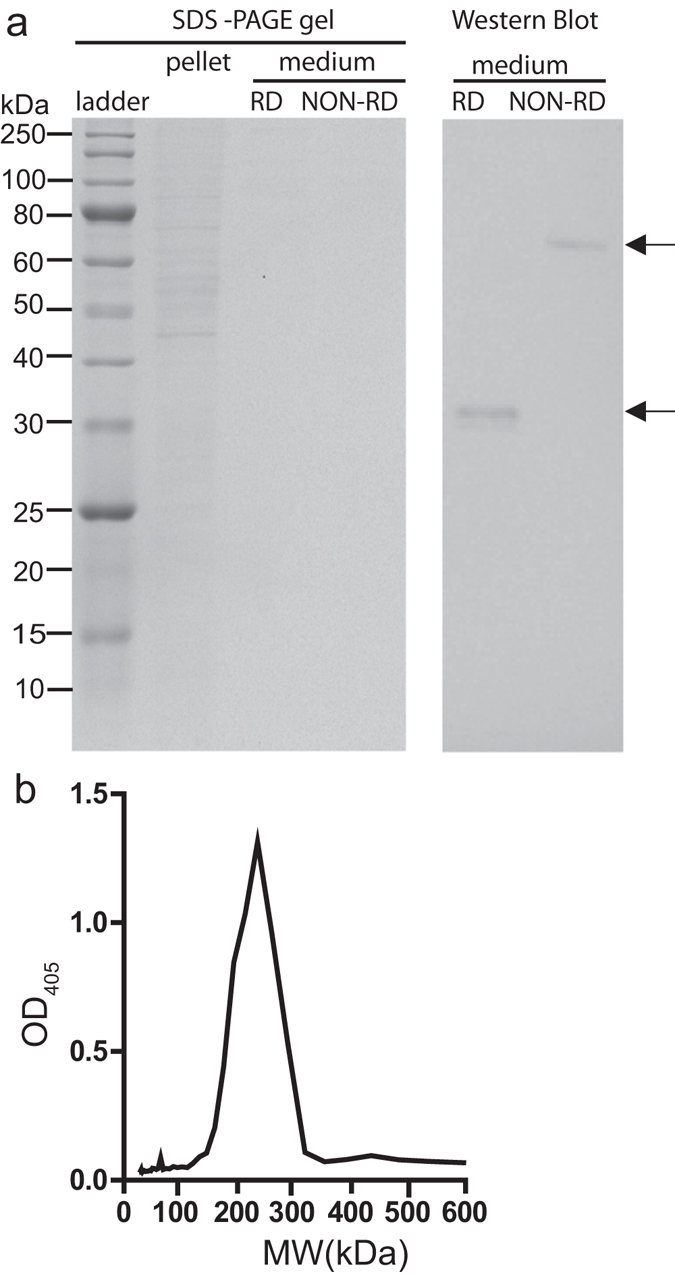
FBN30 is secreted from insect Hi5 cells and forms octamers. (a) The non-reducing (NON-RD) (without β-ME) and the reducing (RD) conditions of 12% SDS-PAGE analysis were performed. FBN30 was detected with anti-FBN30 antibody. A specific band with a molecular mass of ~ 33 kDa (bottom arrow labeled, corresponding to insect cell expressed recombinant FBN30 protein) under reducing condition was detected from the medium (right panel), indicating that the recombinant FBN30 is a secreted protein. Under non-reducing conditions, a specific band of ~66 kDa (top arrow labeled) was detected with anti-FBN30, suggesting that FBN30 forms a homodimer through disulfide bond. (b) The FBN30 protein from the concentrated medium was applied onto a size exclusion chromatography using a Superdex-200 increase column and the FBN30 in each eluted fraction was quantified with ELISA assays. The molecular mass of FBN30 ranged from 212–274 kDa, suggesting that the native FBN30 exists as an octamer, which is formed by four disulfide-bond linked homodimers.
