Figure 1.
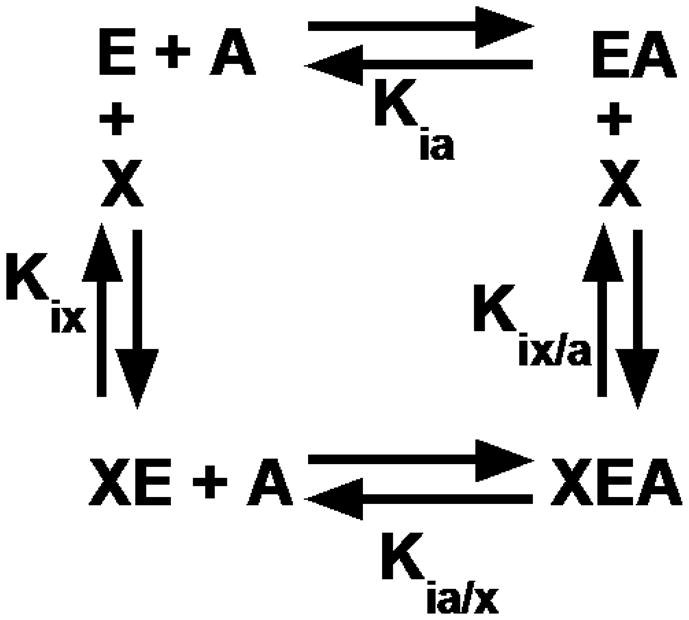
A reaction scheme for an allosteric energy cycle in which an enzyme (E) can bind one substrate (A) and one allosteric effector (X). Kia is the equilibrium dissociation constant of the substrate binding to the enzyme in the absence of effector. Kia/x is the equilibrium dissociation constant of the substrate binding to the enzyme in the presence of saturating concentrations of effector. Kix is the equilibrium dissociation constant of the effector when substrate is absent, while Kix/a is the equilibrium dissociation constant of effector in the presence of saturating concentrations of substrate.
