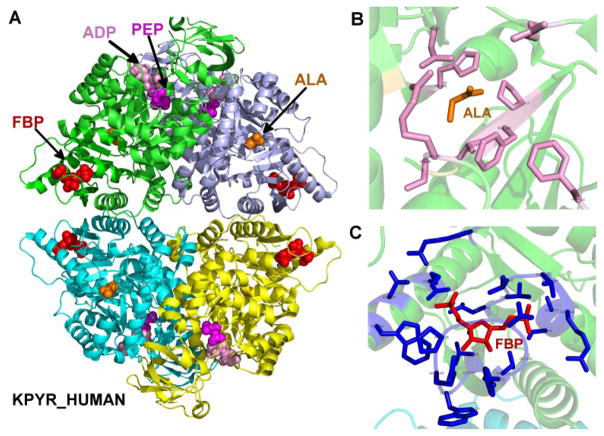
Figure 2.
Structure of human pyruvate kinase, as well as the binding sites of inhibitor alanine and activator fructose-1,6-bisphosphate. (A) A modeled structure of L-PYK tetramer with substrates PEP and ADP, allosteric inhibitor alanine, and allosteric activator. PEP, ADP, alanine (labeled ALA) and fructose-1,6-bisphosphate (labeled FBP) are shown in spheres, colored in magenta, pink, orange and red respectively. The structure was assembled by superposing monomers from several structures of homologues of L-PYK with PEP, ADP, and alanine bound onto a tetrameric structure of human L-PYK with fructose-1,6-bisphosphate bound (PDB: 4IP7). (B). The allosteric binding site of alanine. Alanine is shown in sticks and colored in orange. Residues that were mutated in experiment 1 are shown in sticks, and colored in pink. (C). The binding site of fructose-1,6-bisphosphate (FBP). FBP is shown in sticks and colored in red. Interacting residues are shown in sticks and colored in blue.