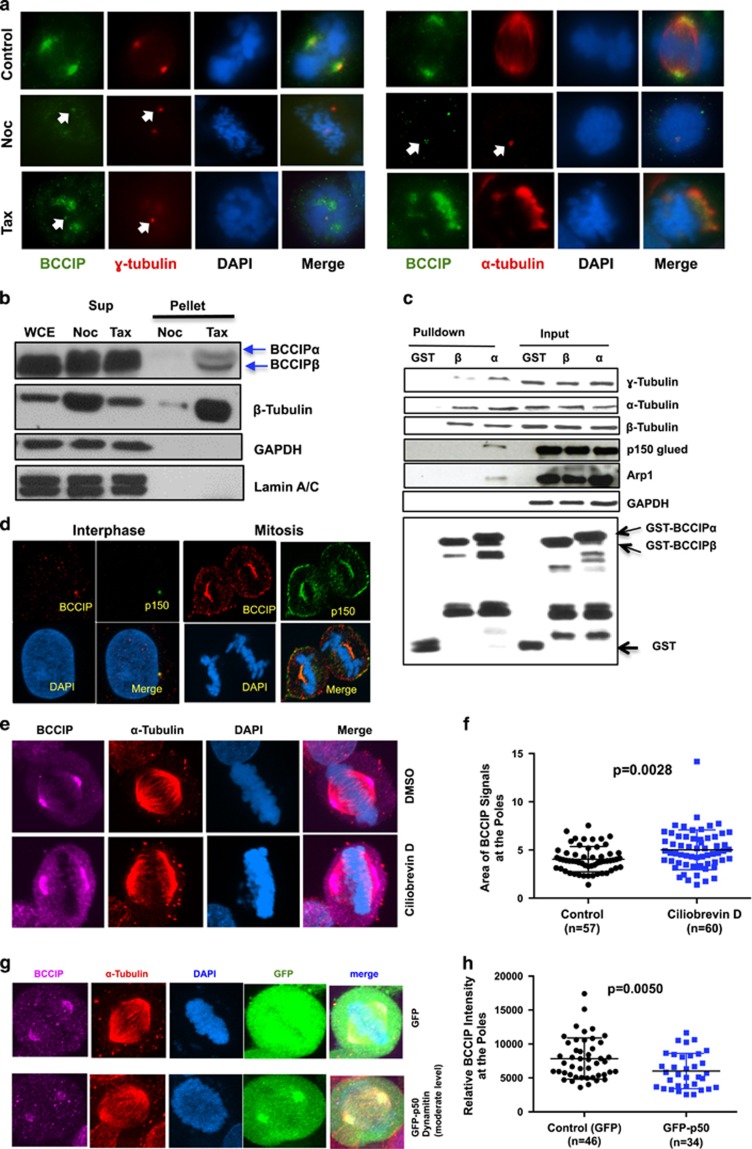
Figure 3.
BCCIP binds to microtubules and is recruited to spindle poles by dynein/dynactin activity. (a) The localization of BCCIP to spindle poles requires microtubules. U2OS cells were treated with nocodazole or taxol and were co-stained with BCCIP and γ-tubulin (left group) or with BCCIP and α-tubulin (right group). When the spindle is depolymerized by nocodazole, α-tubulin stains the nocodazole-resistant centriole microtubules. The arrows indicate the small fraction of BCCIP that remains stably associated with centrosomes following treatments. (b) BCCIP co-precipitates with polymerized microtubules. Mitotic cell lysates were precleared by ultracentrifugation. The soluble lysates were then treated with taxol (Tax) to repolymerize microtubules or nocodazole (Noc) to prevent repolymerization. Following ultracentrifugation, microtubules are in the pellet (P), and soluble tubulin is retained in the supernatant (S). Equal portion of supernatant and pellet fractions were subjected to western blot and with the indicated antibodies. Sup: supernatant; WCE: whole cell extract; N: Nocodazole treated; T: Taxol treated. (c) BCCIP complexes with microtubules, centrosomes and dynactin. HeLa mitotic extract was incubated with GST-BCCIPα, GST-BCCIPβ or GST and subjected to glutathione-bead pulldown. 1% of the input and 5% of the pulldown was subjected to western blot and probed with the indicated antibodies. (d) BCCIP co-localizes with dynactin at the mother centriole, spindle pole and cell cortex. Shown are representative confocal images demonstrating the colocalization of BCCIP with dynactin at the mother centriole during interphase and the spindle pole and cell cortex during mitosis. (e, f) Inhibition of dynein by Ciliobrevin-D disrupts the distribution of BCCIP at the spindle poles. U2OS cells were treated with the dynein inhibitor Ciliobrevin-D and stained with the indicated antibodies. Shown are the representative images (e) and the quantified BCCIP-positive areas from spindle poles (f). (g, h) Overexpression of p50/dynamitin diminishes the levels of spindle pole-associated BCCIP. HeLa cells were transfected with GFP-p50/dynamitin or GFP, and the BCCIP intensity at the poles was quantified. Shown are representative images (g) and quantification of the BCCIP intensity at the poles.