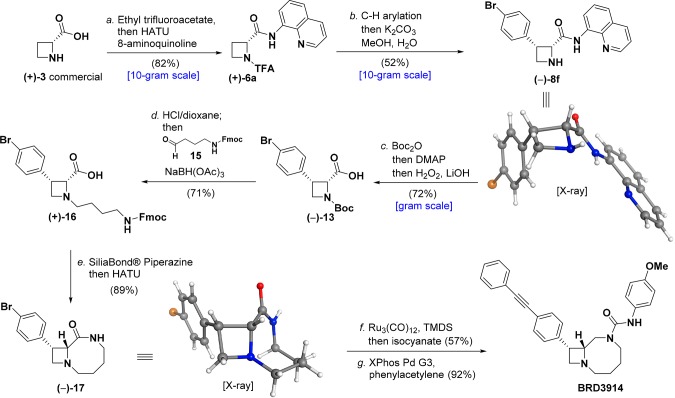
Figure 4.
Reaction conditions: a. Ethyl trifluoroacetate, DIPEA, DMF, 50 °C, 15 h; evaporate volatile materials, then 8-aminoquinoline, HATU, DIPEA, DMF, 0 °C, 2 h (82%); b. Pd(OAc)2 (10 mol %), AgOAc (2.0 equiv), (BnO)2PO2H (20 mol %), 1-bromo-4-iodobenzene (3.0 equiv), DCE, 110 °C, 24 h; evaporate volatile materials, then K2CO3 (3.0 equiv), 9:1 MeOH/H2O (52%); c. Boc2O (3.0 equiv), MeCN, 50 °C, 15 min; then DMAP (0.1 equiv), 2 h; evaporate volatile materials, then H2O2 (10.0 equiv), LiOH (6.0 equiv), THF/MeOH (2:1), 0 °C, then 50 °C, 20 h (72%); d. HCl (4.0 M in dioxane, 15.0 equiv), DCM, 2 h; evaporate volatile materials, then 15 (2.5 equiv), NaBH(OAc)3 (3.0 equiv), DCM, 12 h (71%); e. SiliaBond Piperazine (3.0 equiv), DMF, 50 °C, 2 h; then DIPEA (5.0 equiv), HATU (1.5 equiv), 20 min (89%); f. Ru3(CO)12 (10 mol %), 1,1,3,3-tetramethyldisiloxane (15.0 equiv), toluene, 90 °C, 24 h; then 4-methoxyphenylisocyanate (1.0 equiv), THF, 10 min (57%); g. XPhos Pd G3 (10 mol %), phenylacetylene (5.0 equiv), Et3N (4.0 equiv), MeCN, 70 °C, 2 h (92%).