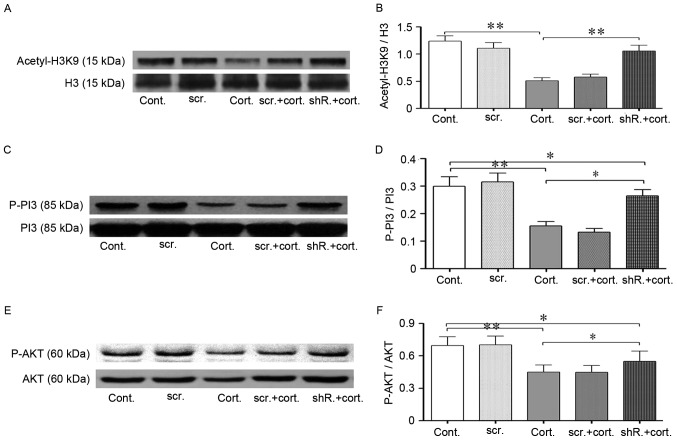
Figure 5.
LV-shRNA-HDAC2 knockdown partially restored the reduced acetylation level of histone H3K9 and increased PI3K-AKT signaling pathway phosphorylation. (A and B) Western blot analysis indicated restored acetylation of H3K9 following lentivirus-shRNA-HDAC2 transfection. Corticosteroid treatment (2 µM, 48 h) significantly decreased the histone acetylation levels of H3K9 in neuro-2a (N2a) cells. HDAC2-shRNA transfected cells exhibited increased histone H3K9 acetylation levels during corticosteroid treatment compared with the untransfected cells in the same condition. Total H3 was used as the loading control. (C-F) HDAC2 knockdown increased PI3K/AKT phosphorylation. (C and D) In the same treatment conditions, the phosphorylation of PI3K p85 in HDAC2-shRNA transfected cells was substantially increased compared with the untransfected cells. Total PI3K was used as the loading control. (E and F) Following corticosteroid incubation, AKT (ser473) phosphorylation in HDAC2-shRNA transfected cells was partially restored compared with the untransfected cells. Total AKT was used as the loading control. Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01 between two groups. At least 3 cultures were used per group. shRNA, short hairpin RNA; HDAC1, histone deacetylase 2; H3K9 histone 3, lysine 9; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; n2a, neuro-2a; cont, control cells; cort, corticosteroid treated cells; scr, scramble shRNA transfected cells; shR, transfected cells.