Abstract
Mangiferin is a polyphenolic compound present in Salacia reticulata. It has been reported to reduce bone destruction and inhibit osteoclastic differentiation. This study aimed to determine whether mangiferin directly affects osteoblast and osteoclast proliferation and differentiation, and gene expression in MC3T3-E1 osteoblastic cells and osteoclast-like cells derived from primary mouse bone marrow macrophage cells. Mangiferin induced significantly greater WST-1 activity, indicating increased cell proliferation. Mangiferin induced significantly increased alkaline phosphatase staining, indicating greater cell differentiation. Reverse transcription-polymerase chain reaction (RT-PCR) demonstrated that mangiferin significantly increased the mRNA level of runt-related transcription factor 2 (RunX2), but did not affect RunX1 mRNA expression. Mangiferin significantly reduced the formation of tartrate-resistant acid phosphatase-positive multinuclear cells. RT-PCR demonstrated that mangiferin significantly increased the mRNA level of estrogen receptor β (ERβ), but did not affect the expression of other osteoclast-associated genes. Mangiferin may inhibit osteoclastic bone resorption by suppressing differentiation of osteoclasts and promoting expression of ERβ mRNA in mouse bone marrow macrophage cells. It also has potential to promote osteoblastic bone formation by promoting cell proliferation and inducing cell differentiation in preosteoblast MC3T3-E1 cells via RunX2. Mangiferin may therefore be useful in improving bone disease outcomes.
Keywords: mangiferin, osteoblast, osteoclast, runt-related transcription factor 2, estrogen receptor β
Introduction
Mangiferin (MGF) is a naturally occurring polyphenolic compound commonly present in Mangifera indica and Salacia reticulata (1,2). MGF has a variety of pharmacological effects, including antiviral and an antioxidant activity (3,4). There have been a number of studies that have used MGF and S. reticulata for the treatment of diabetes mellitus; MGF and S. reticulata reportedly inhibit the activity of α-glucosidase and suppress the mRNA expression of fructose-1,6-bisphosphatase and glucose-6-phosphatase (5,6). The effects of MGF and S. reticulata on bone and cartilage disease have also been reported; S. reticulata leaf ameliorated the symptoms of rheumatoid arthritis (RA) by reducing bone tissue destruction in type II collagen antibody-induced arthritic mice (7). Furthermore, a previous study reported that MGF inhibits osteoclastic differentiation of RAW 264.7 cells (8).
Osteoblasts and osteoclasts have critical roles in bone tissue; bone metabolism is maintained by the balance of bone formation by osteoblasts and bone resorption by osteoclasts. Osteoblast- and osteoclast-associated bone diseases include osteoporosis, osteopetrosis and RA (9–11). RA is also a chronic inflammatory disease, characterized by inflammatory cell infiltration, synovial hyperplasia, and destruction of cartilage and bone (12–14); this bone tissue destruction is induced and promoted by osteoclast activation (15). In addition, osteoblasts produce receptor activator nuclear factor-κB ligand (RANKL) as an osteoclast differentiation factor (16).
RA treatment is currently primarily based on the administration of anti-inflammatory drugs, immunosuppressive drugs and antibody medicines (17–19); calcium, steroids and bisphosphonates have also been used to treat the deconstruction of bone (20–22). In addition, bone resorption can be suppressed and bone formation can be enhanced by estrogen receptors, such as estrogen receptor-α (ERα) and ERβ (23).
It was previously reported that the leaf of S. reticulata ameliorated the symptoms of arthritis in a mouse model of RA, and suppressed cell proliferation and gene expression of matrix metalloproteinase 3, RANKL, cathepsin K and c-fos mRNA in murine synovial cells derived from mice with RA (8). This improvement in RA symptoms was potentially associated with the MGF contained in S. reticulata. The present study examined whether MGF directly affects osteoblast and osteoclast proliferation and differentiation. It was aimed to investigate whether MGF affects cell proliferation, cell differentiation, and gene expression in cultured osteoblasts and osteoclasts.
Materials and methods
Ethics statement
The use of experimental animals was approved, and this study was performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee of Josai University (permit no. H22064; Saitama, Japan).
Cell and culture conditions
MC3T3-E1 cells were purchased from RIKEN Cell Bank (RIKEN BioResource Center, Tsukuba, Japan). The cells were cultured in α-minimal essential medium (MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and penicillin (50 IU/ml). Cells were subcultured every 2nd day using trypsin/EDTA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Osteoclast-like cells (OCL) were derived from the bone marrow of three 8-week-old ddY male mice (Tokyo Laboratory Animals Science. Tokyo. Japan). Bone marrow macrophage (BMM) cells were prepared immediately from the femur and tibia of ddY mice. The BMM cells were induced by macrophage colony stimulating factor (M-CSF) from bone marrow cells. Cell suspensions were plated at 1×104 cells/well in a 96-well plate in α-MEM containing 10% FBS, penicillin, and supplemented with M-CSF (20 ng/ml) and RANKL (10 ng/ml) to induce osteoclast differentiation. The cells were cultured as described previously (24).
Cell proliferation assay
MC3T3-E1 cells were plated in 96-well microplates (BD Biosciences, Franklin Lakes, NJ, USA) at a density of 3×103 cells/well. After 24 h of incubation, MGF (1×10-3, 10–4, 10–5, 10–6 and 10–7 M) dissolved in dimethyl sulfoxide was mixed in the culture medium and added to each well. The control group was treated with medium only. After 2 h, the cell proliferation reagent WST-1 (Roche Diagnostics, Indianapolis, IN, USA) was added to each well (1/10 volume of the previously added medium), and the plates were incubated for 30 min. Cell proliferation was then measured at 450 nm using a spectrophotometer (Wallac 1420ARVO.SX multilabel counter; PerkinElmer, Inc., Waltham, MA, USA). All experiments were performed in triplicate with independent samples.
Alkaline phosphatase (ALP) activity
After 21 days of treatment with MGF, MC3T3-E1 cells were fixed with 100% methanol. The fixed cells were incubated in ALP staining solution for 20 min at 37°C; the ALP staining solution consisted of 0.05 M AMP buffer at pH 9.8, 10 mM naphthol AS-BI phosphate (Sigma-Aldrich; Merck KGaA), and 1 mM fast red violet LB salt (Sigma-Aldrich; Merck KGaA). The ALP stained areas were then inspected using a light microscope (Penguin 600CL; Pixera Corporation, Tokyo, Japan). The ALP activity was quantified by densitometric analysis using ImageJ software (version 1.3; National Institutes of Health, Bethesda, MD, USA). All experiments were performed in triplicate with independent samples.
Tartrate-resistant acid phosphatase (TRAP) activity
After 6 days of culture, the OCL were fixed with 100% methanol. The fixed cells were incubated in the presence of L (+)-tartrate buffer (0.335 mol/l, pH 4.9±0.1) with a TRAP kit (Sigma-Aldrich; Merck KGaA) for 5 min at 37°C. Following staining, TRAP stained areas were inspected using a light microscope (Penguin 600CL). TRAP-positive cells containing three or more nuclei were counted as multinuclear osteoclasts. The TRAP activity was quantified by densitometric analysis using ImageJ software. All experiments were performed in triplicate with independent samples.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from MC3T3-E1, BMM and OCL were extracted from the cultures using TRIzol reagent, and RNA was extracted according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was prepared from 1 µg total RNA using the SuperScript First-Strand Synthesis system for RT-PCR (Invitrogen; Thermo Fisher Scientific, Inc.). Amplification was performed in 10 µl of reaction mixture containing 1 µl cDNA using EX Taq (Takara Bio, Inc., Otsu, Japan). The primer sequences used for each PCR are outlined below. Initial denaturation was performed at 94°C for 30 sec, annealing temperatures ranged from 58–62°C for 30 sec, and extension was done at 72°C for 3 min. A final extension was performed at 72°C for 3 min. PCR cycles varied from 20–60 cycles, due to certain genes, particularly RANK and cathepsin K, exhibiting multiple variations and therefore requiring further cycles in order to confirm gene expression.
Primers were as follows: Cathepsin K, 5′-CCAGTGTGGTTCCTGTTGG-3′ (forward) and 5′-TTGCCGTGGCGTTATACAT-3′ (reverse); ERα, 5′-AATTCTGACAATCGACGCCAG-3′ (forward) and 5′-GTGCTTCAACATTCTCCCTCCTC-3′ (reverse); ERβ, 5′-CAAGCTCATCTTTGCTCCAG-3′ (forward) and 5′-GCAGATGTTCCATGCCCTTG-3′ (reverse); nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), 5′-CACCAAAGTCCTGGAGATCC-3′ (forward) and 5′-GAAACGCTGGTACTGGCTTC-3′ (reverse); NF-κB, 5′-GCTTTGCAAACCTGGGAATA-3′ (forward) and 5′-TCCGCCTTCTGCTTGTAGAT-3′ (reverse); RANK, 5′-CCAGGGGACAACGGAATCAG-3′ (forward) and 5′-GGCCGGTCCGTGTACTCATC-3′ (reverse); osteoclast-associated receptor (OSCAR), 5′-TGTTCTGGAACTGCTGGTAACG-3′ (forward) and 5′-GATGAGGTTTCCCTGGGTATAG-3′ (reverse); runt-related transcription factor 1 (RunX1), 5′-GGTCGTTGAATCTCGCTACC-3′ (forward) and 5′-ACTTCCTCTGCTCCGTGCTA-3′ (reverse); RunX2, 5′-ACACCTACTCTCATACTGGGATGAGGAATG-3′ (forward) and 5′-ATGGTGGAGATCATCGCGGACCACCCGGCC-3′ (reverse); GAPDH, 5′-TTGACCTCAACTACATGG-3′ (forward) and 5′-CAGGGTGGTGGACCTCAT-3′ (reverse).
The PCR products were separated on 2% agarose gel with Tris-acetate-EDTA buffer and visualized with ethidium bromide. All gels were digitally imaged and the band intensities of these digital images were determined using ImageJ software. Each mRNA level was normalized against the GAPDH mRNA level. All data are presented as the fold change in the target/GAPDH ratio. All experiments were performed in triplicate with independent samples.
Statistical analysis
All experiments were performed with triplicate independent samples and were repeated at least three times, giving qualitatively identical results. Statistical analysis was carried out with Stat-Mate III version 3.18 (ATMS Co., Ltd., Tokyo, Japan). Data were analyzed using the Student's t-test, with P<0.05 considered to indicate a statistically significant difference.
Results
Effects of mangiferin on cell proliferation, cell differentiation and gene expression regulation of MC3T3-E1 cells
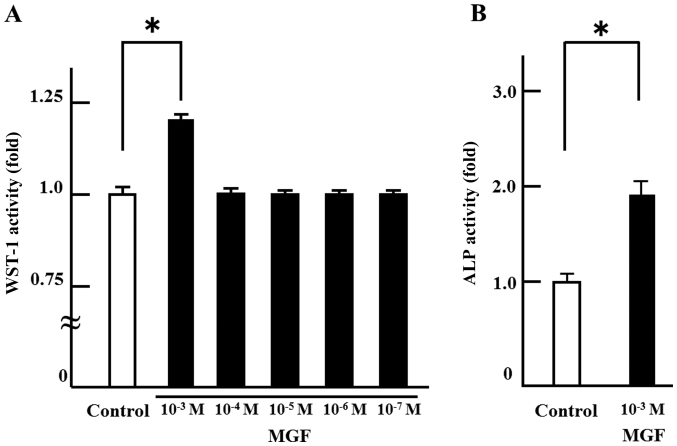
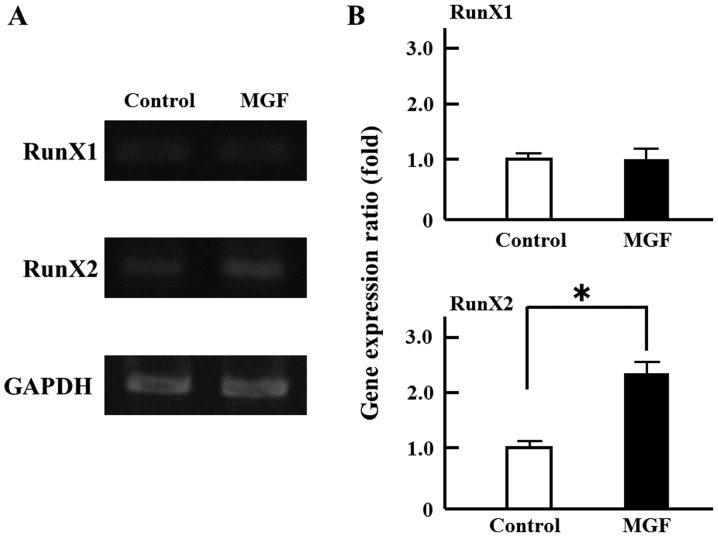
In order to investigate the effect on cell proliferation, MGF was added at concentrations ranging from 10-3 M to 10-7 M. There was no change in cell proliferation with MGF at concentrations of 10-4 M to 10-7 M compared with the control. Only 10-3 M MGF resulted in an increase in WST-1 activity; 10-3 M MGF induced >20% cell proliferation of MC 3T3-E1 cells compared with controls (Fig. 1A). Thus, 10-3 M MGF was selected for use in the subsequent experiments. In order to investigate the effect of MGF on cell differentiation, ALP staining was performed. MGF induced an ~2-fold increase in the ALP-stained area of MC 3T3-E1 cells compared with controls (Fig. 1B). Regarding the effect of MGF on the expression of genes involved in the differentiation of osteoblasts, MGF induced >2-fold increase in RunX2 mRNA expression in MC3T3-E1 cells; however, MGF did not affect the level of RunX1 mRNA expression (Fig. 2A and B).
Figure 1.
Effects of MGF on cell proliferation and differentiation of MC3T3-E1 cells. (A) MC3T3-E1 cells were treated with or without MGF. After 2 h of incubation, cell proliferation was measured by WST-1. (B) MC3T3-E1 cells were treated with or without MGF. After 21 days of incubation, the cells were subjected to ALP staining. The results are expressed as the mean ± standard deviation of three independent experiments (n=3). *P<0.05. MGF, mangiferin; ALP, alkaline phosphatase.
Figure 2.
Effect MGF on gene expression related to osteoblast-regulated genes. (A) Total RNA extracted from MC3T3-E1 cells treated with or without MGF (10−3 M) for 2 h. mRNA expression of RunX1 and RunX2 were analyzed by reverse transcription-polymerase chain reaction. (B) The semi-quantitative results are expressed as the mean ± standard deviation of three independent experiments (n=3). *P<0.05. MGF, mangiferin; RunX, runt related transcription factor.
Effects of mangiferin on cell differentiation and gene expression regulation in osteoclast lineage cells
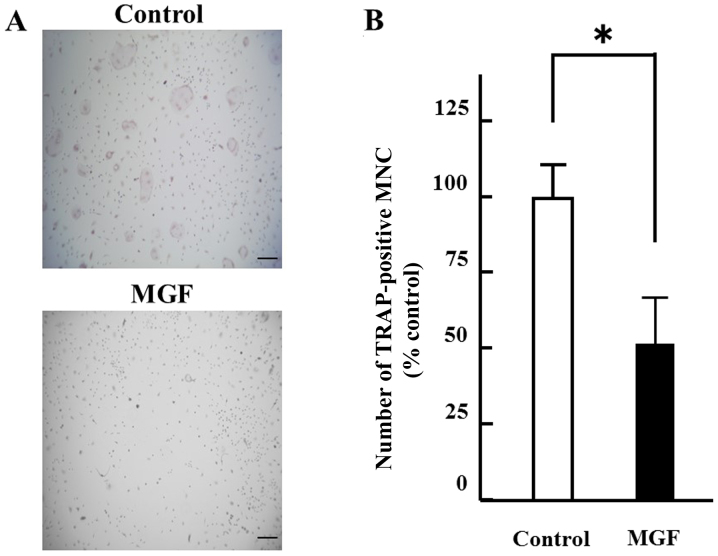
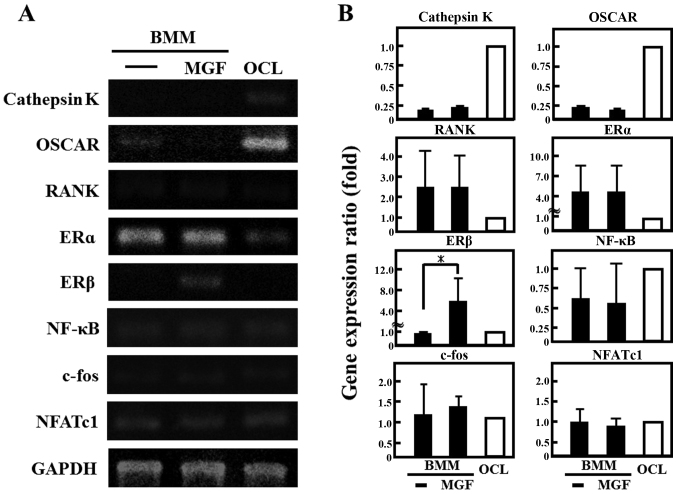
The effect of 10-3 M MGF on osteoclast differentiation was determined. The number of TRAP-positive multinuclear cells were counted. MGF reduced the number of TRAP-positive multinuclear cells by ~50% compared with the vehicle control (Fig. 3A and B). Regarding the effect of MGF on the expression of genes involved in the differentiation of osteoclasts, the cathepsin K and OSCAR mRNA expression in BMM cells was ~1/4 of that in OCLs; RANK was two-fold higher and ERα was 4-fold higher in BMM cells compared with mature OCL cells (Fig. 4A and 4B). In addition, MGF treatment increased the ERβ mRNA expression level in BMM by ~5-fold compared with untreated BMM. However, MGF did not affect the expression levels of cathepsin K, c-fos, ERα, NF-κB, NFATc1, OSCAR or RANK mRNA in BMM.
Figure 3.
Effect of MGF on TRAP-positive MNCs formation from BMM cells. (A) Mouse BMM cells were treated with or without MGF (10−3 M) in the presence of macrophage colony stimulating factor (20 ng/ml) and receptor activator nuclear factor-κB ligand (10 ng/ml). TRAP staining was performed to determine the effect on osteoclast formation. After 6 days of incubation, the cells were subjected to TRAP staining and compared with osteoclast-like cells (control). (B) The results are expressed as the mean ± standard deviation of three independent experiments (n=3). Scale bar, 100 µm; original magnification, ×200. *P<0.05. MGF, mangiferin; TRAP, tartrate-resistant acid phosphatase; MNCs, multinuclear cells; BMM, bone marrow macrophage.
Figure 4.
Effect of MGF on gene expression of osteoclast-associated genes. (A) The mRNA expression of cathepsin K, OSCAR, RANK, ERα, ERβ, NF-κB, c-fos, and NFATc1 were analyzed by reverse transcription-polymerase chain reaction. Total RNA extracted from control OCL in the presence of macrophage colony stimulating factor (20 ng/ml) and RANK ligand (10 ng/ml) for 3 days and BMM cells treated with or without MGF (10−3 M) for 3 h. (B) The results are expressed as the mean ± standard deviation of three independent experiments (n=3). *P<0.05. BMM, bone marrow macrophages; MGF, mangiferin; OCL, osteoclast-like cells; OSCAR, osteoclast-associated receptor; RANK, receptor activator nuclear factor-κB; ER, estrogen receptor; NF-κB, nuclear factor-κB; NFATc1, nuclear factor of activated T-cells, cytoplasmic 1.
Discussion
The present study aimed to investigate whether MGF affects cell proliferation, cell differentiation and gene expression in cultured osteoblasts and osteoclasts. For example, α-mangostin, a compound similar to MGF, has been reported to affect cell proliferation in human lung adenocarcinoma cells and human breast adenocarcinoma cells (25,26). The present study indicated that MGF may promote cell proliferation and cell differentiation of osteoblasts. Furthermore, MGF may induce osteoblast differentiation from preosteoblasts to mature osteoblasts via RunX2. It is established that RunX1 regulates the differentiation of hematopoietic stem cells, and that RunX2 regulates the differentiation of osteoblasts (27,28). Additionally, Runx2 and ALP are validated osteoblastic differentiation markers (29–31). The results of the present study indicated that MGF may inhibit M-CSF- and RANKL-induced osteoclast formation and differentiation from BMM to TRAP-positive multinuclear cells as osteoclasts. These results indicate that MGF suppressed the differentiation of osteoclasts from BMM and promoted the expression of ERβ mRNA in BMM. These data suggest that MGF ay suppress osteoclastogenesis via the ERβ signal. In conventional investigation of osteoclasts, the effect on TRAP-positive cells indicates the induction of differentiation of osteoclasts (32,33). In addition, it is established that ER suppresses osteoclastogenesis (34,35).
In conclusion, MGF promoted cell proliferation and induced cell differentiation in preosteoblast MC3T3-E1 cells via RunX2; thus, MGF may potentially promote osteoblastic bone formation. MGF suppressed cell differentiation to mature OCL, and promoted the mRNA expression of ERβ in BMM; thus, MGF may potentially inhibit osteoclastic bone resorption. MGF may adjust the balance between osteoblast and osteoclast function, and could be useful in improving bone diseases. Further study is warranted into the use of MGF as a treatment for bone diseases such as osteoporosis, osteopetrosis and RA.
References
- 1.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. In mice. J Ethnopharmacol. 2001;78:133–137. doi: 10.1016/S0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa M, Ninomiya K, Shimoda H, Nishida N, Matsuda H. Hepatoprotective and antioxidative properties of Salacia reticulata: Preventive effects of phenolic constituents on CCl4-induced liver injury in mice. Biol Pharm Bull. 2002;25:72–76. doi: 10.1248/bpb.25.72. [DOI] [PubMed] [Google Scholar]
- 3.Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez GM, Re L, Giuliani A, Núñez-Sellés AJ, Davison GP, León-Fernández OS. Protective effects of Mangifera indica L. Extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res. 2000;42:565–573. doi: 10.1006/phrs.2000.0727. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Yang XW, Wang RF. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72:242–247. doi: 10.1016/j.phytochem.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Im R, Mano H, Matsuura T, Nakatani S, Shimizu J, Wada M. Mechanisms of blood glucose-lowering effect of aqueous extract from stems of Kothala himbutu (Salacia reticulata) in the mouse. J Ethnopharmacol. 2009;121:234–240. doi: 10.1016/j.jep.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi Y, Mano H, Nakatani S, Shimizu J, Wada M. Effects of the Sri Lankan medicinal plant, Salacia reticulata, in rheumatoid arthritis. Genes Nutr. 2010;5:89–96. doi: 10.1007/s12263-009-0144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang E, Liu Q, Qi M, Liu HG, Yang X, Chen H, Zheng MH, Xu J. Mangiferin attenuates osteoclastogenesis, bone resorption, and RANKL-induced activation of NF-κB and ERK. J Cell Biochem. 2011;112:89–97. doi: 10.1002/jcb.22800. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009;15:1066–1071. doi: 10.1038/nm.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soysa NS, Alles N, Weih D, Lovas A, Mian AH, Shimokawa H, Yasuda H, Weih F, Jimi E, Ohya K, Aoki K. The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809–818. doi: 10.1359/jbmr.091030. [DOI] [PubMed] [Google Scholar]
- 11.Childress P, Philip BK, Robling AG, Bruzzaniti A, Kacena MA, Bivi N, Plotkin LI, Heller A, Bidwell JP. Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif Tissue Int. 2011;89:74–89. doi: 10.1007/s00223-011-9496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk JL, Cordaro LA, Wei H, Benjamin JB, Yocum DE. Synovium as a source of increased amino-terminal parathyroid hormone-related protein expression in rheumatoid arthritis. A possible role for locally produced parathyroid hormone-related protein in the pathogenesis of rheumatoid arthritis. J Clin Invest. 1998;101:1362–1371. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 14.Steinitz M, Izak G, Cohen S, Ehrenfeld M, Flechner I. Continuous production of monoclonal rheumatoid factor by EBV-transformed lymphocytes. Nature. 1980;287:443–445. doi: 10.1038/287443a0. [DOI] [PubMed] [Google Scholar]
- 15.Ainola M, Li TF, Mandelin J, Hukkanen M, Choi SJ, Salo J, Konttinen YT. Involvement of a disintegrin and a metalloproteinase 8 (ADAM8) in osteoclastogenesis and pathological bone destruction. Ann Rheum Dis. 2009;68:427–434. doi: 10.1136/ard.2008.088260. [DOI] [PubMed] [Google Scholar]
- 16.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 17.Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford) 2005;44:813–818. doi: 10.1093/rheumatology/keh538. [DOI] [PubMed] [Google Scholar]
- 18.Hansen IB, Ellingsen T, Hornung N, Poulsen JH, Lottenburger T, Stengaard-Pedersen K. Plasma level of CXC-chemokine CXCL12 is increased in rheumatoid arthritis and is independent of disease activity and methotrexate treatment. J Rheumatol. 2006;33:1754–1759. [PubMed] [Google Scholar]
- 19.Puéchal X, Miceli-Richard C, Mejjad O, Lafforgue P, Marcelli C, Solau-Gervais E, Steinfeld S, Villoutreix C, Trèves R, Mariette X, et al. Anti-tumour necrosis factor treatment in patients with refractory systemic vasculitis associated with rheumatoid arthritis. Ann Rheum Dis. 2008;67:880–884. doi: 10.1136/ard.2007.081679. [DOI] [PubMed] [Google Scholar]
- 20.Lories R. The balance of tissue repair and remodeling in chronic arthritis. Nat Rev Rheumatol. 2011;7:700–707. doi: 10.1038/nrrheum.2011.156. [DOI] [PubMed] [Google Scholar]
- 21.Luppen CA, Blake CA, Ammirati KM, Stevens ML, Seeherman HJ, Wozney JM, Bouxsein ML. Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid-treated rabbits. J Bone Miner Res. 2002;17:301–310. doi: 10.1359/jbmr.2002.17.2.301. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Mashiba T, Burr DB. Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int. 2001;69:281–286. doi: 10.1007/s002230010036. [DOI] [PubMed] [Google Scholar]
- 23.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 24.Mano H, Hakeda Y, Kumegawa M. Estrogen directly down-regulates the bone-resorbing activity of mature osteoclasts through nuclear estrogen receptor alpha. Cytotechnology. 2001;35:17–23. doi: 10.1023/A:1008188120766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih YW, Chien ST, Chen PS, Lee JH, Wu SH, Yin LT. Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys. 2010;58:31–44. doi: 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA, Wu PF, Shih YX, Shih YW. Alpha-mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma cells. J Food Sci. 2010;75:H13–H23. doi: 10.1111/j.1750-3841.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa M, Goyama S, Asai T, Kawazu M, Nakagawa M, Takeshita M, Chiba S, Ogawa S, Kurokawa M. AML1/Runx1 negatively regulates quiescent hematopoietic stem cells in adult hematopoiesis. J Immunol. 2008;180:4402–4408. doi: 10.4049/jimmunol.180.7.4402. [DOI] [PubMed] [Google Scholar]
- 28.Liu JC, Lengner CJ, Gaur T, Lou Y, Hussain S, Jones MD, Borodic B, Colby JL, Steinman HA, van Wijnen AJ, et al. Runx2 protein expression utilizes the Runx2 P1 promoter to establish osteoprogenitor cell number for normal bone formation. J Biol Chem. 2011;286:30057–30070. doi: 10.1074/jbc.M111.241505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, Koh JT. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48:885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Laflamme C, Curt S, Rouabhia M. Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch Oral Biol. 2010;55:689–701. doi: 10.1016/j.archoralbio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Lim TY, Wang W, Shi Z, Poh CK, Neoh KG. Human bone marrow-derived mesenchymal stem cells and osteoblast differentiation on titanium with surface-grafted chitosan and immobilized bone morphogenetic protein-2. J Mater Sci Mater Med. 2009;20:1–10. doi: 10.1007/s10856-008-3528-9. [DOI] [PubMed] [Google Scholar]
- 32.Teramachi J, Morimoto H, Baba R, Doi Y, Hirashima K, Haneji T. Double stranded RNA-dependent protein kinase is involved in osteoclast differentiation of RAW264.7 cells in vitro. Exp Cell Res. 2010;316:3254–3262. doi: 10.1016/j.yexcr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 34.Windahl SH, Norgård M, Kuiper GG, Gustafsson JA, Andersson G. Cellular distribution of estrogen receptor beta in neonatal rat bone. Bone. 2000;26:117–121. doi: 10.1016/S8756-3282(99)00248-3. [DOI] [PubMed] [Google Scholar]
- 35.Hiyama S, Sugiyama T, Kusuhara S, Uchida T. Evidence for estrogen receptor expression during medullary bone formation and resorption in estrogen-treated male Japanese quails (Coturnix coturnix japonica) J Vet Sci. 2012;13:223–227. doi: 10.4142/jvs.2012.13.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]