Abstract
Background
South Africa has the highest global burden of human immunodefciency virus [HIV]. The study compared the cost-effectiveness of individual and combination HIV preventive strategies against the current rollout of ART and possible ART scale-up.
Methods
Adolescents attending South African schools in 2012 were included in the semi-Markov running annual cycles. The ART and HIV counseling and testing program [comparator] was weighed against the interventions [viz. HIV vaccine, a dual vaccine strategy [HIV and HPV vaccines], oral pre-exposure prophylaxis [PrEP] and voluntary medical male circumcision [VMMC]; and various combinations thereof. Quality-adjusted life years [QALY] determined changes in HIV associated mortality and infections averted. One-way and probabilistic sensitivity analysis determined parameter uncertainty. Discount rates of 3% with a lifetime horizon [70 years] were applied.
Results
Dual vaccination was highly cost-effective strategy [US$ 7 per QALY gained] and averted 29% of new HIV infections. VMMC [US$ 30 per QALY gained] proved more cost-effective than HIV vaccination alone [US$ 93 per QALY gained], though VMMC averted 6% more new infections than the HIV vaccine when considered among male participants. PrEP interventions were the least cost-effective with pharmaceutical and human resource spending driving the costs. Combined dual vaccination and VMMC strategies were a dominant intervention. Strategies involving PrEP were the least cost-effective.
Conclusion
VMMC, HIV vaccination and dual vaccination strategies were more cost-effective than any PrEP strategies. A multi-intervention biomedical approach could avert considerable new HIV infections and present a cost-effective use of resources; particularly where large scale multi-interventional randomized controlled trials are absent.
Keywords: HIV prevention, Cost-effectiveness analysis, Vaccination, PrEP, VMMC
Introduction
South Africa remains the unenviable epicenter of the human immunodeficiency virus [HIV] pandemic amassing 18% of the global prevalence of disease and forcing heightened efforts by the government to quell the disease burden [1]. The national HIV counseling and testing [HCT] campaign launched in 2010 was a major initiative increasing the numbers of people that had ever been tested by over 15% between 2008 and 2012 [2]. In fact,modeling estimates suggest that universal implementation of HCT among South Africans aged 15 years and older would translate to a 1% reduction in prevalence over the next 50 years [3]. As of 2010, 98% of public health facilities were able to offer prevention of mother to child transmission [PMTCT] of HIV services which reduced annual infections to 2.2% in 2013/2014 [4]. Further, South Africa has developed the most established condom distribution program globally with 506 million male condoms distributed in 2013/2014 alone [4]. Acquired immune deficiency virus [AIDS] education has been successfully integrated into the primary and high school curriculums via a Life Skills Education Program, which aimed at averting new infections while providing support to those children already living with HIV. Lastly, South Africa has managed to orchestrate the largest antiretroviral therapy [ART] rollout program in the world accounting for a third of new ART drug recipients globally between 2010 and 2013 [1]. Despite this, 58% of South Africans eligible for ART treatment remain unable to access it [1]. These significant strides made by government to alleviate the HIV burden have been undermined by a persistently high HIV incidence rate over the years (South Africa accounted for 16% of the global incidence of HIV in 2013) [1].
Global advances in biomedical interventions in the last decade have forced clinicians and decision makers alike to strongly reconsider their HIV prevention packages. Observations from clinical trials investigating voluntary male medical circumcision [VMMC] [5-8] and oral pre-exposure prophylaxis [PrEP] [9-12] have demonstrated partial success in decreasing sexual transmission of HIV. The general consensus by several experts remain that a ‘multi-faceted combination prevention’ approach is critical to curbing the advancement of the epidemic [13-15]. Clinical trials involving combination interventions are rarely investigated due to financial, ethical and feasibility constraints; yet are crucial to our understanding of defining an optimal portfolio of prevention. In the South African context, we considered the cost-effectiveness of implementing PrEP, HIV and human papillomavirus [HPV] vaccinations and VMMC against scaling-up the current coverage of ART.
The encouraging findings of several HIV PrEP trials and the observed 96% reduction in HIV transmission associated with early ART use in sero-discordant couples [HPTN 052] have endorsed the legitimacy of the PrEP intervention and stimulated critical policy discussions regarding the use of ART in HIV prevention [16,17]. The first significant findings were reported among men and transgender women who had sex with men in the iPrEX trial, which yielded a 44% reduction in HIV acquisition with a daily dose of tenofovir/emtricitabine [TDF/FTC] [11]. In two large trials, PrEP was found to be equally effective [between 63% to 73%] in reducing heterosexual HIV transmission in the Partners PrEP conducted in Kenya and Uganda [TDF or TDF/FTC daily] and TDF2 [TDF/FTC daily] studies conducted in Botswana [10,12]. However, similar results were not reproducible in the South African setting asFEM-PrEP [recruiting hetero sexual women in South Africa, Tanzania, and Kenya for daily TDF/FTC] and the oral arm of the VOICE trial [recruiting women in South Africa, Uganda, and Zimbabwe] were closed prematurely for futility [18]. The VOICE trial stopped its gel arm when it became evident that daily gel use was safe but not effective [19]. The CAPRISA 004 trial [a proof of concept study supporting peri-coital vaginal microbicide application] enjoyed short lived success when the use of a 1% TDF vaginal gel decreased HIV-1 incidence by 39% [20]. The FACTS 001 study, conceived to provide supporting data for microbicide licensure in South Africa, was found to be ineffective alluding once more to poor adherence patterns and bringing into question the future use of this prevention modality [19,21]. Despite these reservations, oral PrEP is being introduced in many developing countries including South Africa and due consideration must be given to how this intervention will fit in financially, programmatically and politically [22-24].
Evidence from observational data [25-27] and three randomized controlled trials conducted in Kenya, South Africa and Uganda [6-8] prove conclusively that circumcised men have a significantly reduced risk of acquiring HIV infection. Apart from the strong epidemiological evidence base, the biological basis for the protective effects of VMMC against HIV is deemed plausible [28] and the cost-effectiveness, or even cost-saving, of the intervention has been reinforced by several studies [5,29-31]. In addition to the public health benefits of a HIV prevention strategy reporting high efficacy [approximating 60%], VMMC is a one-time medical procedure with partial but potentially durable effects [32]. The coverage rates for VMMC still remain surprisingly low, with 2.7% coverage reported in a sample of 14 sub-Saharan Africa countries [33]. Heeding this call, South Africa had conducted 3.3 million VMMCs by 2012 [2]. Countries continuing to report low circumcision rates have extensive scope to draw the benefits of the intervention going forward [24,34].
Vaccines are recognized as the most cost-effective intervention in healthcare. Despite several earlier HIV vaccine setbacks [35,36], Rerks-Ngam [2009] announced the first vaccine regimen [RV144/ Thai trial] to show moderate vaccine efficacy in human populations [37]. The HIV vaccine regimen, after being optimized by undergoing modification to make it Clade C specific and changing the adjuvant and protein, entered Phase I clinical trials assessing safety and immunogenicity at six major South African centers under the umbrella of the HVTN 100 study [38]. Costing information regarding its implementation into the expanded program of immunization would present a key advocacy tool to decision makers should this vaccine reach fruition [39,40]. For the purposes of this study, we are considering dual implementation of the HIV vaccine with the HPV vaccine. The HIV-HPV link has long been established. HIV acquisition is enhanced in the presence of cervico-vaginal HPV disease [41,42] and HPV detection is known to drastically increase post HIV sero-conversion [43]. Clinically, the HIV-HPV relationship manifests as rapidly progressive disease [44], resulting in significant associated mortality and morbidity that remains a major concern to the national health department. Given the close relationship the pathologies share, it is not inconceivable that the HPV vaccination may play a role in reducing HIV transmission on the biological basis HPV infection increases the acquisition of HIV infection [45]. South Africa has the highest cervical cancer incidence and mortality globally [46], and the HPV vaccine is already being implemented to a proportion of school-going female learners [47].
Implementing the most efficient portfolio of interventions requires due consideration for the comparative costs and benefits of alternative strategies. Economic evaluation of these interventions individually and in combination can improve our understanding of potential synergistic effects [48], without the financial implications of multi-intervention clinical trials. Additionally, cost-effectiveness analysis can guide decision makers in the efficient allocation of restricted health budgets. Few studies in the past have considered evaluating multiple interventions [49], most concentrating rather on individual interventions. None have evaluated the use of the HIV vaccine. The aim of this study is to economically evaluate individual and combination HIV preventive strategies and compare their impact against both the current rollout of ART and a potential scaling-up of the ART program.
Materials and Methods
The study methodology was compliant with the reporting guidelines of the Consolidated Health Economic Evaluation Reporting Standards [CHEERS] statement [50].
Study overview
Adolescents attending South African schools in 2012 were considered for the evaluation. The programs were considered for implementation in schools based on the national health initiative to develop school-based sexual and reproductive health services [51], with the intention of targeting learners prior to their sexual debut, thus preventing HIV acquisition. A lifetime horizon, that was considered congruent with current life expectancy in South Africa, was modeled. The health service provider [provider] perspective was adopted. The data generated would be used to review of health service delivery and decision making; and explore the financial implications of introducing these health interventions in the public sector. The interventions were modeled as prevention strategies that reduced the HIV disease burden and associated mortality. The interventions were considered against the system of HCT and the national rollout of ART that constituted the standard of care in South Africa [52]. The current coverage achieved by the national ART program was estimated at 29% [1]. Economic costs and health outcomes were discounted at a rate of 3% , with an uncertainty range of 0% to 6%, as recommended by the World Health Organization Choosing Interventions that are Cost-Effective [WHO-CHOICE] guidelines [53].
Outcome measures
Choice of health outcomes
The EuroQol EQ-5D health outcome measurement tool has been validated for measuring health related quality of life [HRQOL] of HIV/AIDS in Africa, hence validating the use of the quality-adjusted life year [QALY] in the South African health context [54]. The QALY combines survival and HRQOL into a single health summary measure informing decision-making regarding the relative value for money of health care interventions [55]. The estimation of the QALY is derived from calculating the total life-years gained from an intervention, then multiplying each year by a quality of life score, where 0 = worst health and 1 = best health, thus reflecting the quality of life achieved in that year [56]. The incremental cost-effectiveness ratio [ICER] of the interventions compared to the standard of care was measure using Equation 1:
| (1) |
Where C1 and E1 are the costs and effects of the comparator [the current standard of care], and C2 and E2 are the costs and effects of the intervention.
HIV/AIDS pooled utilities derived from a meta-analysis, were used for the cost-utility analyses of HIV related interventions [57]. The utility weights for HIV disease appears in (Table 1).
Table 1. HIV-related utility weights.
| Parameters | Estimate | Source |
|---|---|---|
| Full health | 1 | |
| HIV disease | ||
| Asymptomatic | 0.94 | (57) |
| Symptomatic | 0.82 | (57) |
| AIDS | 0.70 | (57) |
The table describes the health related quality of life weights for the different HIV related health states where full health carries a weight of one (1)
Study inputs
For the purposes of demonstrating the impact of the dual HIV and HPV vaccine strategy, input parameters concerning both these diseases were assessed in the model (Table 2). Only costs related to HIV services were included in the model. The model was constructed and parameterized using transition probabilities obtained from South African published sources. The study considered costs adjusted to the common year 2012. Costs were converted from South Africa rand [ZAR] to the United States dollar [US$] for ease of international comparison, using the average exchange rate for 2012 at ZAR 8.21 to the US$ 1 [58]. The standard HIV treatment was assumed to be delivered by primary health care [PHC] nurses consulting patients, while complicated cases would be up-referred to doctors or medical specialists. Pharmaceutical costing included ART, treatment of sexually transmitted infections [STI] and condom delivery. Additionally, the comparator cost was added to the intervention [vaccine and vaccine delivery] as both services would run concurrently in the intervention group. Laboratory tests pricing were obtained from the National Health Laboratory Services [NHLS] and medication, consumables and additional pharmaceuticals and valuations of medical personnel costs were derived from the Uniform Patient Fee Schedule [UPFS] sourced from the National Department of Health. These parameters are detailed in Table 3.The annual estimated costs of implementing the interventions included human resources [e.g. consultation with a PHC nurse and counselor]; pharmaceuticals [e.g. drugs, STI treatment and condoms] and laboratory costs [e.g. regular HIV testing, creatinine monitoring and pregnancy testing] – as stipulated by the relevant guidelines.
Table 2. Model parameters pertaining to the study population.
| Parameters | Base-case estimate | Reference |
|---|---|---|
| Vaccine characteristics | ||
| Coverage | 60% | assumption |
| HPV vaccine efficacy | 70% | (59) |
| HIV vaccine efficacy | 50% | assumption |
| Treatment uptake | ||
| Cervical screening | 13.6% | (46) |
| ARV therapy | 29.0% | (1) |
| HPV treatment | 35.3% | (60) |
| Transition probabilities (represented as percentages) | ||
| HIV negative | ||
| Development of LSIL | 3.00 | (44) |
| Progression of LSIL to HSIL | 1.69 | (44) |
| Progression of HSIL to cancer | 3.84 | (60) |
| HIV positive | ||
| HIV incidence in general population | 2.28 | (2) |
| HIV incidence in HPV disease | 5.39 | (44) |
| Development of LSIL | 14.00 | (61, 62) |
| Progression of LSIL to HSIL | 6.00 | (61, 62) |
| Progression of HSIL to cancer | 8.10 | (44) |
| Mortality | ||
| Mortality in general population | 1.16 | (63, 64) |
The possibility of transition from one health state to the next is described. The estimates were obtained from relevant South African literature for the year 2012.
Table 3. Unit cost of screening, diagnosis and treatment of HPV disease in 2012 (US$).
| Economics | Value | Range | Reference |
|---|---|---|---|
| Cost | 3.0% | (0 – 6%) | (53) |
| Outcome | 3.0% | (0 – 6%) | (53) |
| International comparison (ZAR: 1US$) | ZAR 8.21 | - | (58) |
| HIV disease related costs | Distribution | Value | Reference |
| HIV program | |||
| HIV vaccine | - | 12 | assumption |
| Vaccine delivery per dose* | Gamma | 17 | (65-69) |
| Existing prevention program (incl. HR) | Gamma | 65 | (66-70) |
| Voluntary counseling and testing (VCT) (per test) | Gamma | 23 | (66, 67) |
| Cost of HIV rapid testing | Gamma | 2 | (66, 67) |
| ARV treatment | Gamma | 310 | (67, 70, 71) |
| Not on ARV | Gamma | 65 | (70) |
| Other HIV prevention interventions | Distribution | Value | Reference |
| HPV vaccine | - | 17 | (72) |
| Vaccine delivery per dose | Gamma | 17 | (65, 67) |
| Annual PrEP cost | Gamma | 140 | (73) |
| Voluntary medical male circumcision | Gamma | 79 | (74) |
The estimates were obtained from relevant South African literature for the year 2012.
Initial course comprises 6 doses
Interventions considered-HIV vaccine
There is no commercially available HIV vaccine, thus characteristics modeled were hypothetical. The vaccine characteristics were determined by the target product profile suggested by the Pox-Protein Private-Public Partnership [P5] research collaboration established to build on the foundations of the RV144 / Thai trial [75]. The regimen included in this economic evaluation mirrored the on-going HVN 100 study which adapted the ALVAC prime ALVAC/gp120 boost of the RV144/ Thai trial but added an additional ALVAC/gp120 boost at month 12. This boost at month 12 was intended to circumvent the waning of the immune response documented a year following initial vaccine administration in the RV144/Thai trial. Apivotal phase IIb/III HIV vaccine efficacy trial is scheduled for implementation in South Africa shortly. The study, designated HVTN 702, will evaluate the same regimen [as HVTN 100].
The HIV vaccine coverage estimated 60% receiving the initial course. This was a roughly based on the 68% coverage achieved for the 3rd dose of diphtheria toxoid, tetanus toxoid and pertussis vaccine [DTP3] [76].The DTP3 coverage has been validated as a proxy for immunization system strength and performance nearly globally in the recent decades [77]. The coverage range was then explored in the sensitivity analysis. The hypothetical base-case HIV vaccine scenario estimated a vaccine cost of US$ 12 per dose, vaccine efficacy of 50% [Range: 30 - 70%] and vaccine duration of protection spanning 10 years [achieved through annual booster administration]. The declining immunity demonstrated in the RV144 / Thai trial following the first year of administration necessitated the need for booster injections. The model countered the declining immunity by adopting the conservative approach of annual boosters. While this may not represent a pragmatic solution, it merely translated to an overestimation of costs in the context of an economic evaluation. The HIV vaccine price of US$ 12 was based on the public sector pricing of the HPV vaccine pricing [US$ 17]. Markedly reduced vaccines prices were deemed attainable considering the great strides made in the public sector in negotiating lower priced ART and HPV vaccines [71,78] and given the extensive disease burden in the country. The price assumption was tested in the sensitivity analysis. The HIV vaccine characteristics are hypothetical and based on the target product profile described by the P5, based on data available from major HIV vaccine clinical studies conducted thus far [75]. The bivalent HPV vaccine was modeled as it is being administered in lower socio-economic schools as part of the government initiative [79]. The negotiated vaccine price was US$ 17 per dose and efficacy was determined by the documented clinical trials of the vaccine [59]. The HPV vaccine course was completed over 2 years and was administered concomitantly with the HIV vaccine to achieve vaccine coverage of 60%. The HPV vaccine was assumed to confer lifelong protection [80]. Delivery of health services was conducted at the schools. Relevant HIV related cost components were identified from the 2013 national treatment guideline adopted in the South African public healthcare sector [52].
HPV vaccine
The implementation of the HPV vaccine was considered in this study considering the synergistic relationship HPV disease shares with HIV disease [61,62]. Reporting the highest disease burden for both these diseases, it makes programmatic sense for South Africa to address these diseases simultaneously. Progression to cervical cancer is drastically increased in the presence of HIV infection and the bivalent vaccine [Cervarix® [GlaxoSmithKline Biologicals, Rixensart, Belgium]] targets the VLP types 16 /18, that have been implicated in 64% of cervical cancer is South Africa [46]. The vaccine was considered at 60% coverage, but limited to the female population only.
Voluntary medical male circumcision
VMMC has been definitively proven to reduce female to male sexual transmission of HIV by 60% [Range: 28 – 66%] [6-9]. The findings of these landmark studies compelled the WHO to launch an unprecedented public health drive in 2009 calling for 80% coverage of voluntary, safe, culturally appropriate and affordable male circumcision by 2016 [6]. South Africa responded to the initiative by performing150 000 VMMCs by April 2011, averting 1 HIV infection for every 5 procedures done [81]. However, drawbacks in the South Africa programarose as the procedures could only be performed by doctors and the service was marred by the poor quality of facilities and surgical care [82]. The introduction of PrePex in South Africa [an elastic ring device requiring no local anesthetic that can be placed and removed by a mid-level health care worker] held the promise of accelerating the rollout while alleviating the workload placed on limited numbers of healthcare workers. VMMC is highly cost-effective [74]. Being a once-off procedure that has potential benefit for the rest of his life, VMMC holds is a significant player in the fight against HIV in South Africa [74]. The model assumed coverage of 60%, and this was tested in the sensitivity analysis. Costing involved the PrePex system, a cheaper procedure for VMMC that negated the need for surgical procedures [74].
Pre-exposure prophylaxis
Antiretroviral chemoprophylaxis has shown great promise in preventing HIV acquisition. The iPrEx study [2010] demonstrated the initial encouraging work in this field with a 44% reduction in HIV incidence noted among males having sex with males [MSM] receiving daily doses of TDF. This convincing data formed the basis for the South African guideline describing the standard of care for MSM antiretroviral chemoprophylaxis in South Africa [9]. The model used in this study uses this guideline to determine the costs implicated in a national PrEP program. In 2011, a landmark study, HPTN 052, showed early initiation of antiretroviral treatment [in people with a CD4 count between 350 and 550 cells/mm3] for the HIV-positive partner in a serodiscordant couple reduced HIV transmission to the HIV-negative partner by 96% [16]. Oral PrEP [Truvada, a combination of TDF/FTC] has been approved for use in South Africa since late 2015, though the indications for use and the extent of the rollout have yet to be properly defined. The monthly cost of the drug used in this analysis was determined by the current tender price that the drug is available to the South African government [US$ 6.32 per month in 2012] [73]. Oral PrEP, not vaginal microbicidal formulations, was considered in the analysis. The study assumed coverage of 60%, with effectiveness of 67% [Range: 44 – 81%] for high adherence and 21% [Range: -31 – 52%] for low adherence [10-12,83]. Lower price estimates, coverage and effectiveness measures were assessed in the sensitivity analysis.
Antiretroviral therapy
The model considered the HIV prevention interventions discussed against the current rollout of ART [29% coverage] [1] and against a potential increase in the rollout to cover the 58% treatment gap cited by UNAIDS [1].
Assessing combination interventions
The combined effectiveness of two interventions [such as VMMC and PrEP] has rarely been assessed in a clinical trial setting. In the absence of concrete data, mathematical modeling techniques are used to determine the combined effectiveness under different assumptions [49]. The methodology for the calculation of the combined intervention effectiveness was adopted from a similar study conducted by Long and Stavert [49]. They suggested that the efficacy may be multiplicative [e.g. if VMMC is assumed to be 60% effective and PrEP 67% effective, then the combined effectiveness would be calculated as in Equation 2:
| (2) |
Model based economic evaluation
The data capture and analysis was conducted in Microsoft Excel® [Version 2010] [Microsoft Corp., Redmond, WA]. Ersatz version 1.2 [www.epigear.com], a boot-strap add-in application for Microsoft Excel, was used to perform the uncertainty analysis. The core model developed was a semi-Markov simulation with annual cycles (Figure 1). Semi-Markov models were considered in this evaluation as it allowed for the addition of tunnel states that counter the ‘memory less’ nature of the Markov model and permitted the modeling of recurrent disease episodes. The study population started the model disease free and each year was exposed to the risk of acquiring either HPV or HIV disease with the rate of disease acquisition adjusted for based on which primary infection was acquired. The model was built using socio-demographic data of the proportion of the population that accesses public health care in South Africa [84]. The study aggregated simulated data of individuals representing a 68.8% coverage rate, the estimated access rate to PHC services [84]. The dual vaccination program was specifically offered to female learners on a voluntary basis. Where males were being considered the impacts of HPV disease / HPV vaccination were omitted. A proportion of healthy individuals [coverage] would be vaccinated against HIV and HPV disease, while the rest remained unvaccinated. Annually, healthy individuals were exposed to the risk of acquiring each disease.
Figure 1.

Semi-Markov model for HPV and HIV related disease states. Healthy individuals (vaccinated and unvaccinated) may remain uninfected or transition into HPV or HIV disease. Those acquiring HPV disease are at risk of acquiring HIV disease (if unvaccinated against HIV). Each state may progress to death during any cycle at a rate dependent on the disease state they were currently in. (LSIL – low grade intra-epithelial neoplasia; HSIL – high grade intra-epithelial neoplasia)
Where the dual vaccination strategy was being considered, healthy individuals could acquire HPV disease from a low grade intra-epithelial lesions [LSIL] to high grade intra-epithelial lesions, which has the potential to progress to cervical cancer. At each proposed HPV related health state, an individual may die [not represented graphically] or acquire HIV infection [which would see them progress on the lower ‘HIV positive’ spectrum of disease]. HIV positive, HPV infected individuals that were treated for HPV disease could potentially develop recurrent HPV disease. The model allowed for females with treated and untreated HPV disease to acquire HIV infection. There was a greater risk of transition to more serious HPV states among those HIV positive vs. those HIV negative. HIV positive individuals could potentially enter the HIV treatment pool. Every health state, irrespective of disease status, could progress to death at a rate determined by their current health state with consideration to the background mortality independent of their current health state. Mortality transitions were excluded from (Figure 1) as they rendered the model excessively ‘bushy’ and concealed the key message of the diagram. The arrows represented the transition probabilities from one state to another, with costs and utility measures then added to each health state to predict costs and QALYs over the 70 year duration of the intervention and the comparator. Once the HIV vaccine had been stopped, the HIV event rates were assumed lower in the HPV vaccinated individuals compared with the HPV unvaccinated individuals [61,62]. HPV vaccine protection was considered lifelong [80].
One-way sensitivity analyses were conducted to evaluate the impact of single assumptions on cost and health outcomes. Probabilistic sensitivity analysis [PSA] performed with a bootstrapping technique was used to explore the uncertainty in the model and evaluate the robustness of the results. These results were presented as cost effectiveness scatter plots. The PSA data generated was used to determine if the intervention fell below the willingness-to-pay [WTP] threshold. As South Africa does not have a pre-defined WTP threshold, the Gross Domestic Product [GDP] per capita [2012] was used as a proxy in accordance with the WHO Guide to Cost-Effective Analysis [53,85]. The WTP threshold was thus defined as US$ 7 508 [ZAR 61 641] per QALY gained. Given the lack of sensitivity of the GDP as a measure of cost-effectiveness, a benchmark intervention was additionally used as a threshold established by analysis of existing practice [86]. In the case of South Africa, VMMC has been established as a cost effective intervention in several analyses [81,87,88].To validate the finding of this analysis, and the cost-effectiveness was assessed against both the GDP and the benchmark intervention.
The study participants were considered sexually naïve at the start of the model. The model assumed that children eligible for schooling were indeed attending school, and that the consent obtained from parents was reflected in the coverage rates. The efficacy of dual interventions was considered multiplicative [49] as there is rarely data obtained from clinical trials reporting the efficacy of two prevention interventions simultaneously introduced. The model confirmed to the principle of global uptake and provision of HCT in schools as stipulated by the national policy [89]. The exercise modeled the rollout of HIV preventive interventions under the umbrella of the comprehensive school-based program to be delivered to all learners, irrespective of socio-economic status. Lastly, the model assumed relatively high uptake of school-based health services given that the provision of care occurs in a safe and familiar environment, without impacting negatively on school attendance. There have been no formal studies to validate this assumption.
Ethical consideration
Ethical approval for the study was obtained from the Human Research Ethics Committee [Medical] of the University of the Witwatersrand.
Results and Discussion
Single interventions
The implementation of individual HIV prevention interventions was compared with the cost of ART in the public sector (Table 4).The coverage of ART in 2012 [status quo] was considered at 29% and including HCT being offered at facilities [1]. Scaling up the coverage of the current ART program to 58% to meet the demands of the existing deficit in South Africa does result in a minimal decrease in mortality but is vital to improve the health of those already infected with the virus. However, this improvement in health comes at a sizeable cost and marginal improvement in QALY as the intervention influences incidence indirectly. The same can be said of the HPV vaccine which shares a synergistic relationship with HIV, and reduces HIV incidence indirectly [61,62]. The health effects of the HPV vaccine and HIV vaccine administered in combination to women is marked. There is a reduction in HIV incidence, HIV associated mortality and the intervention is significantly cost-effective at US$ 7.02 per QALY gained. Individually, the HIV vaccine and VMMC has similar health benefits to the dual vaccine initiative, but the VMMC project is more cost-effective given that it is a once-off procedure that provides the protection. PrEP is an expensive option as well, with an ICER of US$ 257.31 per QALY gained. By the standards of the WHO CHOICE, every intervention would be deemed cost-effective as the ICER value are below the GDP defined threshold of cost-effectiveness [US$ 7 508]. The ICER for VMMC could also serve as a proxy to benchmark intervention cost-effectiveness in South Africa, as it has been the validated through several independent research studies in South Africa as a cost-effective medical intervention [86]. By virtue of this benchmark, only the dual vaccination strategy would then be deemed cost-effective. The dual vaccine strategy offered the largest gain in health benefits for US$ 7 per QALY gained.
Table 4. Health outcomes and cost-effectiveness of individual HIV prevention interventions.
| HIV infection (over 10 years) (%) | Incremental | |||||
|---|---|---|---|---|---|---|
| Incidence | Infections averted | Deaths | QALYS | COST | CER | |
| Status quo | 5.44 | - | 0.43 | 12.53 | 6467.96 | - |
| Upscale ART | - | - | 0.42 | + 0.56 | + 461.27 | 823.21 |
| HIV vaccine | 4.28 | 21.24 | 0.34 | + 4.80 | + 445.41 | 92.77 |
| HPV vaccine | 5.30 | 2.54 | 0.41 | + 3.68 | + 488.20 | 132.53 |
| Dual vaccine | 3.95 | 29.00 | 0.30 | + 14.30 | + 100.42 | 7.02 |
| VMMC | 3.97 | 27.39 | 0.32 | + 12.52 | + 379.50 | 30.32 |
| PrEP | 3.79 | 30.24 | 0.31 | + 13.04 | + 2 247.83 | 172.41 |
Single interventions are compared with the status quo (i.e. continuing to treat HIV infection with ART at 29% coverage (1)).
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
(ART scale-up covers the ART deficit of 58%; HIV and HPV vaccine quoted at 50% and 70% vaccine efficacy
The graphical representation of these results displays the dual vaccine strategy to be the most economically efficient strategy (Figure 2). The price of the PrEP intervention is inherently undetermined at this point, but the assumption made reflects a rather optimistic scenario: markedly reduced pricing [US$ 220 per annum] with high adherence [67%] and high coverage [60%]. Despite this, the implementation of PrEP remained one of the least cost-effective strategies, even at the current low tender price in South Africa.
Figure 2. Cost effectiveness analysis for the individual HIV prevention interventions.

Discounted incremental costs and QALYS over 10 years are displayed for the single interventions
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
Sensitivity analysis of single interventions
A one-way sensitivity analysis demonstrates the impact of varying a single parameter on the overall cost-effectiveness [90]. Table (5) identified the markedly improved ICER outcomes associated with VMMC and the dual vaccine strategy compared with the implementation of the HIV vaccine alone or with an intervention that involved the use of PrEP. Unsurprisingly, decreased cost and increased intervention effectiveness and coverage were associated with improved ICER values across all interventions. The higher discount rate, at 6%, was also associated with a greater ICER value. This could be explained by the investment for the intervention being made now [present costs] with benefits only being realized at a later date [future implications].
Table 5. Scenario analyses compared with base findings.
| HIV Vaccine | Dual vaccine | PrEP | VMMC | |||
|---|---|---|---|---|---|---|
| Discount rate | 0% | Net cost | 4.81 | 14.28 | 13.06 | 12.54 |
| QALYs gained | 447.38 | 84.42 | 2 236.88 | 370.65 | ||
| ICER | 93.05 | 5.91 | 171.22 | 29.56 | ||
| 6% | Net cost | 4.80 | 14.31 | 13.03 | 12.51 | |
| QALYs gained | 446.76 | 107.58 | 2 254.96 | 385.58 | ||
| ICER | 93.10 | 7.52 | 173.05 | 30.82 | ||
| Cost | High | Net cost | 4.80 | 14.30 | 13.04 | |
| QALYs gained | 532.55 | 193.14 | 2 247.83 | n/a | ||
| ICER | 110.92 | 13.50 | 172.41 | |||
| Low | Net cost | 4.80 | 14.30 | 13.04 | ||
| QALYs gained | 399.66 | 51.73 | 1 723.95 | n/a | ||
| ICER | 83.24 | 3.62 | 132.23 | |||
| Very low | Net cost | 4.80 | 14.30 | 13.04 | ||
| QALYs gained | 373.12 | 23.48 | 1 400.79 | n/a | ||
| ICER | 77.71 | 1.64 | 107.44 | |||
| Effectiveness | High | Net cost | 4.69 | 15.03 | 13.04 | |
| QALYs gained | 426.61 | 99.47 | 2 253.37 | n/a | ||
| ICER | 90.97 | 6.62 | 172.83 | |||
| Low | Net cost | 4.92 | 13.61 | 10.19 | ||
| QALYs gained | 464.96 | 101.02 | 1 869.09 | n/a | ||
| ICER | 94.58 | 7.42 | 183.5 | |||
| Coverage | High | Net cost | 5.60 | 16.68 | 15.21 | 14.60 |
| QALYs gained | 486.72 | 84.23 | 2 557.83 | 371.64 | ||
| ICER | 86.89 | 5.05 | 168.16 | 25.45 | ||
| Low | Net cost | 2.40 | 7.15 | 6.52 | 6.26 | |
| QALYs gained | 321.49 | 148.99 | 1 339.99 | 403.06 | ||
| ICER | 133.92 | 20.84 | 205.55 | 64.41 |
One way sensitivity analysis was done to systematically examine the impact of selected variables in the analysis by varying it across a plausible range of values with other variables remaining at their baseline level.
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
Multiple interventions
The evaluation of combined interventions is shown in Table 6.Simultaneously increasing the ART coverage and adding another recognized HIV prevention intervention results in a reduction in HIV incidence exceeding 50%. Increasing the ART coverage is imperative in addressing the already existing burden of HIV disease in South Africa. The synergism between the interventions in decreasing the HIV incidence rates was noted. Interventions involving PrEP had significantly higher cost implications and thus higher ICER values. The implementation of VMMC in combination with the HIV vaccine proved more cost-effective than the implementation of VMMC with an increased ART coverage. The only combination of interventions that resulted in cost-saving employed the use of the dual HIV and HPV vaccination strategies in combination with the VMMC rollout.
Table 6. Health outcomes and cost-effectiveness of combination HIV prevention interventions.
| HIV infection (over 10 years) (%) | Incremental | |||||||
|---|---|---|---|---|---|---|---|---|
| Incidence | Infections averted | Deaths | QALYS | Cost | CER | |||
| Status quo | 5.44 | - | 0.43 | 12.53 | 6467.96 | - | ||
| Scenario | Combinations | ART | ||||||
| VMMC | Upscale | 2.56 | 52.49 | 0.31 | + 13.13 | + 361.10 | 27.50 | |
| VMMC + HIV vaccine | Current | 3.46 | 36.39 | 0.28 | + 18.24 | + 206.52 | 11.32 | |
| Upscale | 2.25 | 58.71 | 0.27 | + 18.87 | + 581.11 | 30.80 | ||
| VMMC + PrEP | Current | 3.24 | 40.35 | 0.27 | + 30.78 | + 2 062.08 | 67.00 | |
| Upscale | 2.11 | 61.28 | 0.26 | + 31.42 | + 2 433.94 | 77.46 | ||
| HIV vaccine + PrEP | Current | 3.15 | 41.99 | 0.26 | + 19.02 | + 2 188.01 | 115.08 | |
| Upscale | 2.06 | 62.11 | 0.25 | +19.65 | + 2 563.53 | 130.42 | ||
| Dual + VMMC | Current | 3.04 | 44.11 | 0.24 | + 42.68 | Dominant | Dominant | |
Combined interventions are compared with the status quo (i.e. continuing to treat HIV infection with ART at 29% coverage (1))
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
(ART scale-up covers the ART deficit of 58%; HIV and HPV vaccine quoted at 50% and 70% vaccine efficacy respectively; VMMC efficacy is 60%, PrEP efficacy is 67% (Range = 21% - 67%); all coverage =60%.)
The graphical representation of the combined interventions is displayed in Figure 3.Interventions involving PrEP is shown to involve a significantly higher incremental cost. Combinations involving VMMC and the HIV vaccine demonstrate a larger gain in QALYS and are associated with lower incremental cost increases. The introduction of the dual vaccine strategies in combination with VMMC proved to be the most economically efficient strategy, increasing QALYs while decreasing costs, relative to the status quo. The strategy is the only cost-saving one.
Figure 3. Cost effectiveness analysis for the combination HIV prevention interventions.
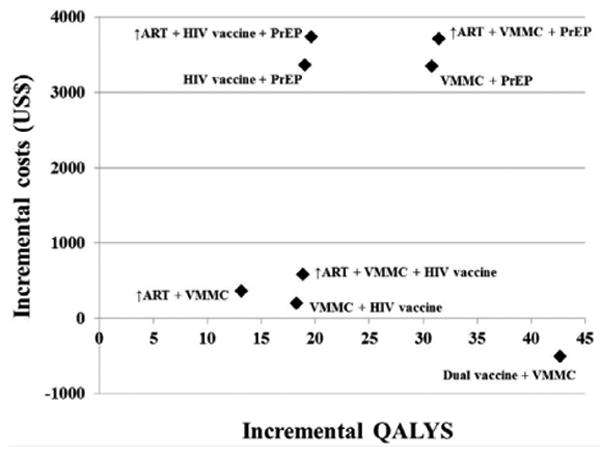
Discounted incremental costs and QALYS over 10 years are displayed for combined interventions
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
Probabilistic sensitivity analysis
The interventions included under combinations 1, 2 and 3 are associated with improved health outcomes at a greater cost compared with the current standard of HIV care in the public sector. Bootstrapping analysis was conducted by repeated sampling [1000 iterations] to estimate the model uncertainty regarding costs and effects (Figure 4). The majority of the iterations lying in the north east [NE] quadrant of the cost-effectiveness [CE] plane [an area of trade-off indicating greater health gain for added expenditure] raises the critical issue of determining how much a decision maker is prepared to pay for an additional unit gain in health outcome. The limited vertical variation indicates limited variability associated with treatment costs. The reported ICERs for all three interventions included in combinations 1, 2 and 3 remained well below the WTP threshold and were thus deemed cost-effective. The iterations of combination 4 appear in the SE quadrant implying treatment dominance i.e. the elements of combination 4 implemented concurrently would prove to be a more effective and less costly [cost-saving] measure.
Figure 4. Cost effectiveness plane for combination HIV prevention interventions.
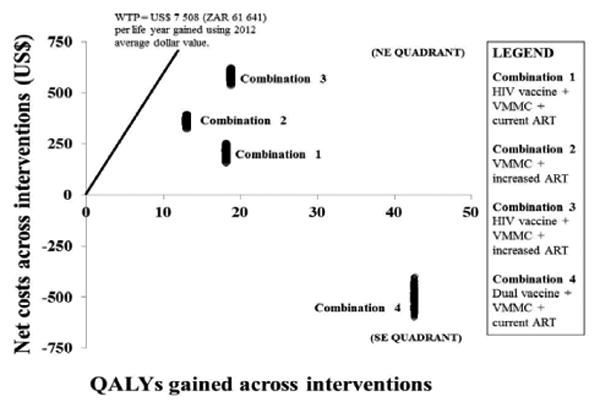
Incremental costs and effects were graphically demonstrated on the incremental cost-effectiveness plane. The x-axis represents the plane according to incremental cost (positive above, negative below), while the y-axis represents the plane according to incremental effect (positive to the right, negative to the left), thus dividing the incremental cost-effectiveness plane into 4 quadrants through the origin. Values falling below the WTP threshold indicated are cost-effective
ART: Antiretroviral Therapy; HIV: Human Immunodeficiency Virus; HPV: Human Papillomavirus; Dual Vaccine: HIV and HPV Vaccinations; VMMC: Voluntary Male Medical Circumcision; Prep: Pre-Exposure Prophylaxis
Discussion
In 2014, it was reported that nearly 2 million people were newly infected with HIV globally [91]. Internationally, public health programs are still unable to sufficiently curb the HIV incidence more than 25 years into the epidemic. This study aimed to generate insights into potential biomedical HIV prevention strategies regarding their cost and health implications when implemented individually and in combination. Previous modeling studies confirm that no single option can curtail the epidemic but rather, a portfolio of complementary prevention and treatment options designed around the specific needs of specific populations should be sought [92]. Biomedical interventions specifically, comprise chemical and physical strategies targeting biological and physiological processes responsible for HIV acquisition and transmission [93].
The HIV vaccine was found to be cost-effective and biologically feasible, averting 21% of new infections in a 10 year period. Work done by Harmon et al. showed a similar reduction in HIV incidence in low-middle income countries from 2.0 million in 2014 to 550,000 in 2070 [92]. The HIV vaccine proved cost-effective even at lower coverage rates and at lower effectiveness rates in the sensitivity analysis. This is important when considering that even a partially effective vaccine could contribute to a sustainable response to HIV/AIDS [94]. However, the issues of cost become critical in developing countries as cost-effectiveness estimates are sensitive to market prices, uptake of services and intervention efficacy [95]. The HIV vaccine in combination with the HPV vaccine resulted in a considerably improved ICER of US$ 7 per QALY gained. Considering the burden of HPV disease in South Africa, this finding is particularly relevant implying the indirect effect of the HPV prevention strategy on HIV acquisition [46,61,62].
Scaling-up of ART coverage has been deemed throughout the study to be independently imperative, but still represents a significant financial investment. Apart from preventing the progression to AIDS, ART can reduce the number of new infections by decreasing the amount of circulating virus in the body of an infected individual [16]. In 2014, UNAIDS' proposed the “90-90-90” campaign where90% of all people living with HIV know their HIV status, 90% of those HIV-positive people who know their status receive ART, and 90% of all people receiving ART have achieved and sustained viral suppression by 2020 [96]. However, ART distribution extends beyond mere provision of ART drugs. ART access and adherence represents logistical, financial and behavioral challenges between people knowing their status and achieving complete viral suppression, even in high income countries [97] Recent updated guidance by the WHO recommends initiation of ART at the time of a positive HIV diagnosis, and 15.8 million people living with HIV [less than half of the total] accessing treatment by June of 2015 [92]. However, it was noted in this study and others that scaling up ART programs provided greater value than untargeted PrEP programs [22]. Further to this, the implementation of a PrEP program could never supersede increasing ART coverage to those individuals already infected with HIV.
PrEP has been shown to reduce HIV infection among men who have sex with men [MSM] in several large clinical trials and in clinical implementation and a PrEP demonstration project [98]. Despite the optimism of ‘real world’ data, the financial implications of introducing PrEP remains concerning, even at lower prices considered. The price of the drug has greatly impacted the affordability of PrEP programs, and is vital in determining cost-effectiveness [99,100] apart from the drug price, validity and utility ofestimates must encompass the service costs associated including regular blood monitoring and potential drug toxicity and development of resistance [18]. User adherence and potential impacts on other prevention mechanisms such as condom use represents other major concerns [101]. As a relatively new intervention in South Africa, careful consideration has to be given in marketing the intervention, including limited provider knowledge [102]. In the South African context, where HIV stigmatization is rife, the association between PrEP and high risk behavioral practices should not be underestimated as an implementation challenge [103-106]. This highlights the need for solid pre-implementation counseling that addresses PrEP education, myth reduction, potential social prejudices and an accurate assessment of patient obligations as standard [103,107]. Ultimately however, as these study findings concur, PrEP remains a relatively low value alternative in the general population despite its demonstrated effectiveness [22]. From an economic perspective, the findings of this study demonstrate that the implementation of a PrEP program may result in significant opportunity costs.
Overall, the use of the HIV vaccine with the HPV vaccine [females] and VMMC [males] proved to be the only dominant strategy. Comparatively, the use of VMMC proved more cost-effective as an individual intervention compared with the HIV Vaccine, alluding to the impact the pricing of the vaccine may have going forward. The use of the dual vaccine approach in females makes sense. Apart from the highest HPV incidence and mortality in the world, the HIV incidence in young women and girls in sub-Saharan Africa is twice that of their male counterparts [92]. Similarly among males, VMMC has been demonstrated to be a high cost-effective, highly effective HIV prevention strategy in randomized controlled trials and in cost-effectiveness studies conducted in areas with generalized epidemics [7,32,93,108]. In fact several studies have shown VMMC to be cost-saving due to moderate implementation costs, high and durable protective effects, and the resulting averted HIV care costs [32,109]. Further, unlike most other HIV prevention strategies, VMMC is a once off procedure conferring potentially lifelong protection with no compelling evidence of increased sexual risk taking reported in circumcised men [7,8,93,109-111].
Several limitations were identified in this study. Firstly, models are generally simplifications of reality, an approximation of the true nature. As such much of the detail is simplified to aide understanding and computation. A glaring example of this would be the over-simplification of the complex sexual networking patterns that exist in South African society [112,113]. Secondly, affordability of an intervention must be discerned from its cost-effectiveness. Most interventions, as demonstrated with PrEP and scaling up of ART services, require a substantial financial investment. The analysis presented are unable to predict the availability of the resources to implement such interventions but rather to highlight the options that present a greater return on investment. Thirdly, the analysis considered the South African population as a whole considering the generalized state of the HIV epidemic in South Africa. Stratification by risk groups [MSM or commercial sex workers for example] may potentially yield differing results even in a high prevalence setting. The scenario differs in low prevalence settings where the risk of contracting HIV among the higher risk groups mentioned could be up to 12-19 times higher than in the general population [92]. Fourthly, the potential complications of the interventions and its associated costs were not accounted for. These included complications of VMMC [including infections and surgical complications] and the development of drug resistance from the use of PrEP. Fifthly, with the development of any economic model is the ensuing problem of parameter uncertainty. It is unclear if trial-derived data will differ from those documented in actual clinical practice. Frequently, factors such as risk compensation and adverse events impact negatively on the cost-effectiveness of an intervention. As more data is made available in the literature, particularly in the case of the HIV vaccine, the model would have to be restructured and refined. Lastly, it should be remembered that the value of the ZAR had essentially halved as a currency by 2015/2016 implying that costs are much greater in 2015/2016 than previously.
Conclusion
This analysis does not purport to provide the perfect combination of biomedical HIV prevention strategies applicable in the South African setting. On the contrary, this body of work was intended to stimulate thought and decision making on potential HIV prevention research, intervention options and funding opportunities for delivery mechanisms. The allocation of limited financial and human resources for HIV control measures in South Africa is a priority. It is the findings of this study that adopting a multi-intervention biomedical approach could avert a significant proportion of new HIV infections and present a more cost-effective use of resources, particularly in the absence of large scale multi-interventional randomized controlled trials.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases [NIAID] U.S. Public Health Service Grants UM1 AI068614 [LOC: HIV Vaccine Trials Network] as part of the South African HVTN AIDS Vaccine Early Stage Investigator Program [SHAPe].The support of the DST-NRF Centre of Excellence in Epidemiological Modelling and Analysis towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to SACEMA.
Abbreviations
- AIDS
Acquired Immune Deficiency Virus
- ART
Antiretroviral Therapy
- HIV
Human Immunodeficiency Virus
- HPV
Human Papilloma Virus
- Prep
Oral Pre-Exposure Prophylaxis
- QALY
Quality Adjusted Life-Year
- VMMC
Voluntary Medical Male Circumcision
References
- 1.Joint United Nations Programme on HIV/AIDS. The Gap Report. Geneva,Switzerland: UNAIDS; 2014. [Google Scholar]
- 2.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey 2012. Cape Town, South Africa: HSRC Press; 2014. [DOI] [PubMed] [Google Scholar]
- 3.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematic model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 4.Massyn N, Day C, Peer N, Padarath A, Barron P, English R. District Health Barometer 2013/14. Durban: South Africa Health Systems Trust; 2014. [Google Scholar]
- 5.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;15:3362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 8.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 9.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 13.Folkers GK, Fauci AS. Controlling and ultimately ending the HIV/AIDS pandemic: a feasible goal. JAMA. 2010;304:350–351. doi: 10.1001/jama.2010.957. [DOI] [PubMed] [Google Scholar]
- 14.Hankins CA, de Zalduondo BO. Combination prevention: a deeper understanding of effective HIV prevention. AIDS. 2010;24:70–80. doi: 10.1097/01.aids.0000390709.04255.fd. [DOI] [PubMed] [Google Scholar]
- 15.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8:62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doblecki-Lewis S, Kolber MA. Preventing HIV infection: pre-exposure and postexposure prophylaxis. IUBMB Life. 2014;66:453–461. doi: 10.1002/iub.1286. [DOI] [PubMed] [Google Scholar]
- 18.Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401. doi: 10.1371/journal.pmed.1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees H, Delany-Moretlwe S, Lombard C, Baron D, Panchia R, Myer L, et al. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. Conference on Retroviruses and Opportunistic Infections; 23-26 February 2015; Seattle, Washington. 2015. [Google Scholar]
- 20.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alistar SS, Grant PM, Bendavid E. Comparative effectiveness and cost-effectiveness of antiretroviral therapy and pre-exposure prophylaxis for HIV prevention in South Africa. BMC Med. 2014;12:46. doi: 10.1186/1741-7015-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewell BL, Cremin I, Pickles M, Celum C, Baeten JM, Delany-Moretlwe S, et al. Estimating the cost-effectiveness of pre-exposure prophylaxis to reduce HIV-1 and HSV-2 incidence in HIV-serodiscordant couples in South Africa. PLoS One. 2015;10:e0115511. doi: 10.1371/journal.pone.0115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bärnighausen T, Bloom DE, Humair S. Economics of antiretroviral treatment vs. circumcision for HIV prevention. Proc Natl Acad Sci U A. 2012;109:21271–21276. doi: 10.1073/pnas.1209017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten JM, Richardson BA, Lavreys L, Rakwar JP, Mandaliya K, Bwayo JJ, et al. Female-to-male infectivity of HIV-1 among circumcised and uncircumcised Kenyan men. J Infect Dis. 2005;191:546–553. doi: 10.1086/427656. [DOI] [PubMed] [Google Scholar]
- 26.Drain PK, Halperin DT, Hughes JP, Klausner JD, Bailey RC. Male circumcision, religion, and infectious diseases: an ecologic analysis of 118 developing countries. BMC Infect Dis. 2006;6:172. doi: 10.1186/1471-2334-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halperin DT, Bailey RC. Male circumcision and HIV infection: 10 years and counting. Lancet. 1999;354:1813–1815. doi: 10.1016/S0140-6736(99)03421-2. [DOI] [PubMed] [Google Scholar]
- 28.Uthman OA, Popoola TA, Uthman MM, Aremu O. Economic evaluations of adult male circumcision for prevention of heterosexual acquisition of HIV in men in sub-Saharan Africa: a systematic review. PLoS one. 2010;5:9628. doi: 10.1371/journal.pone.0009628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA. 2008;300:1674–1684. doi: 10.1001/jama.300.14.1674. [DOI] [PubMed] [Google Scholar]
- 30.Mills E, Cooper C, Anema A, Guyatt G. Male circumcision for the prevention of heterosexually acquired HIV infection: a meta-analysis of randomized trials involving 11,050 men. HIV Med. 2008;9:332–335. doi: 10.1111/j.1468-1293.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 31.Moses S, Plummer FA, Bradley JE, Ndinya-Achola JO, Nagelkerke NJ, Ronald AR. The association between lack of male circumcision and risk for HIV infection: a review of the epidemiological data. Sex Transm Dis. 1994;21:201–210. doi: 10.1097/00007435-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS Med. 2006;3:517. doi: 10.1371/journal.pmed.0030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Progress in scale-ip of male circumcision for HIV prevention in Eastern and Southern Africa: Focus on service delivery. Geneva, Switzerland: 2011. [Google Scholar]
- 34.Williams BG, Lloyd-Smith JO, Gouws E, Hankins C, Getz WM, Hargrove J, et al. The potential impact of male circumcision on HIV in Sub-Saharan Africa. PLoS Med. 2006;3:262. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchbinder SP, Mehrotra DV, Fitzgerald DW, Mogg R, Li D, Gilbert PB. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomized, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 38.Liefman LS. NIH-Sponsored HIV Vaccine Trial Launches in South Africa - Early Stage Trial Aims to Build on RV144 Results. National Institute of Allergy and Infectious Diseases. 2015 [Google Scholar]
- 39.Jacob M, Bradley J, Barone MA. Human papillomavirus vaccines: what does the future hold for preventing cervical cancer in resource-poor settings through immunization programs? Sex Transm Dis. 2005;32:635–640. doi: 10.1097/01.olq.0000179892.78342.79. [DOI] [PubMed] [Google Scholar]
- 40.Ault K, Reisinger K. Programmatic issues in the implementation of an HPV vaccination program to prevent cervical cancer. Int J Infect Dis. 2007;11:26–28. doi: 10.1016/S1201-9712(07)60018-6. [DOI] [PubMed] [Google Scholar]
- 41.Averbach SH, Gravitt PE, Nowak RG, Celentano DD, Dunbar MS, Morrison CS, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24:1035–1042. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith-McCune KK, Shiboski S, Chirenje MZ, Magure T, Tuveson J, Ma Y, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PloS one. 2010;5:10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke B, Chetty R. Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer. Mol Pathol. 2002;55:19–24. doi: 10.1136/mp.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Wright TC, Denny L, Kuhn L. Rapid rise in detection of human papillomavirus (HPV) infection soon after incident HIV infection among South African women. J infect dis. 2011;203:479–486. doi: 10.1093/infdis/jiq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houlihan CF, Larke NL, Watson-Jones D, Smith-McCune KK, Shiboski S, Gravitt PE, et al. AIDS. 2012;26:2211–2222. doi: 10.1097/QAD.0b013e328358d908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruni L, Barriouevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, et al. Human Papillomavirus and Related Diseases in South Africa. ICO Information Centre on HPV and Cancer (HPV Information Centre) 2015 [Google Scholar]
- 47.Botha MH, Richter KL. Cervical cancer prevention in South Africa: HPV vaccination and screening both essential to achieve and maintain a reduction in incidence. S Afr Med J. 2015;105:33–34. doi: 10.7196/samj.9233. [DOI] [PubMed] [Google Scholar]
- 48.Excler JL, Rida W, Priddy F, Gilmour J, McDermott AB, Kamali A, et al. AIDS vaccines and preexposure prophylaxis: is synergy possible? AIDS research and human retroviruses. 2011;27:669–680. doi: 10.1089/aid.2010.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long EF, Stavert RR. Portfolios of biomedical HIV interventions in South Africa: a cost-effectiveness analysis. J Gen Intern Med. 2013;28:1294–1301. doi: 10.1007/s11606-013-2417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11:1478–7547. doi: 10.1186/1478-7547-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Department of Health. Provincial Guidelines for the Implementation of the Three Streams of PHC Re-engineering. Pretoria,South Africa: National Department of Health; 2011. [Google Scholar]
- 52.National Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: National Department of Health; 2013. p. 2013. [Google Scholar]
- 53.Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, et al. Making choices in health: WHO Guide to Cost Effectiveness analysis. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 54.Robberstad B, Olsen JA. The health related quality of life of people living with HIV/AIDS in sub-Saharan Africa - a literature review and focus group study. Cost Eff Resour Alloc. 2010;8:5. doi: 10.1186/1478-7547-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scuffham PA, Whitty JA, Mitchell A, Viney R. The use of QALY weights for QALY calculations: a review of industry submissions requesting listing on the Australian Pharmaceutical Benefits Scheme 2002-4. Pharmacoeconomics. 2008;26:297–310. doi: 10.2165/00019053-200826040-00003. [DOI] [PubMed] [Google Scholar]
- 56.Sinanovic E, Moodley J, Barone MA, Mall S, Cleary S, Harries J. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine. 2009;27:6196–6202. doi: 10.1016/j.vaccine.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002;22:475–481. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 58.The World Bank
- 59.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 60.Knegt Y. Audit of cervical cancer screening and colposcopy attendance in rural South Africa. Afr J Reprod Health. 2014;18:70–78. [PubMed] [Google Scholar]
- 61.Adler DH, Kakinami L, Modisenyane T, Tshabangu N, Mohapi L, De Bruyn G, et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26:1645–1652. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omar T, Schwartz S, Hanrahan C, Modisenyane T, Tshabangu N, Golub JE, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: a prospective cohort. AIDS. 2011;25:87–94. doi: 10.1097/QAD.0b013e328340fd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Statistics South Africa. Mid-year population estimates 2014. Pretoria, South Africa: Statistics South Africa; 2014. [Google Scholar]
- 64.Statistics South Africa. Mortality and causes of death in South Africa 2012 Findings from death notification. Pretoria, South Africa: Statistics South Africa; 2014. [Google Scholar]
- 65.National Department of Health. HM08 2013SYR: The supply and delivery hypodermic syringes, needles and bloodletting devices to the Department of Health for the period 01 December 2013 to November 2015. Pretoria, South Africa: National Department of Health; 2013. [Google Scholar]
- 66.National Department of Health. HM09 2014RTK: Supply and delivery of rapid test kits to the Department of Health for the period 1 April 2014 to 31 March 2017. Pretoria, South Africa: National Department of Health; 2014. [Google Scholar]
- 67.National Department of Health. Approved UPFS 2014 Fee Schedule for Externally Funded Patients Treated at Differentiated Amenities (Private Wards at Public Health Care Facilities) Pretoria, South Africa: National Department of Health; 2014. [Google Scholar]
- 68.National Department of Health. HM01-2012CNDM: Supply and delivery of male and female condoms to the Department of Health from 1 December 2012 to 30 November 2014. Pretoria, South Africa: National Department of Health; 2012. [Google Scholar]
- 69.National Department of Health. HP03-2013FP: Supply and delivery of family planning agents to the Department of Health for the period 1 October 2013 to 30 September 2015. Pretoria, South Africa: National Department of Health; 2013. [Google Scholar]
- 70.National Health Laboratory Services. State pricing catalogue 2013. Pretoria, South Africa: 2013. [Google Scholar]
- 71.Kardas-Nelson M, Goswami S. Upping the competition. NSP review. 2013 [Google Scholar]
- 72.National Department of Health. Essential Drugs Programme. Pretoria: 2012. [Google Scholar]
- 73.National Department of Health. HP13-2015ARV: Supply and delivery of anti retroviral medicines to the Department of Health for the period 1 April 2015 to 31 March 2018. Pretoria, South Africa: 2015. [Google Scholar]
- 74.Kim HY, Lebina L, Milovanovic M, Taruberekera N, Dowdy DW, Martinson NA. Evaluating the cost of adult voluntary medical male circumcision in a mixed (surgical and PrePex) site compared to a hypothetical PrePex-only site in South Africa. Glob Health Action. 2015;8:29116. doi: 10.3402/gha.v8.29116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Russell N, Marovich M, editors. P5 Global Access Committee RSA Summit; October 2015. Cape Town International Conference Centre; P5 Update and GAC Progress Report. [Google Scholar]
- 76.World Health Organisation. Annual WHO/UNICEF Joint Reporting Form and WHO Regional office reports (Updates of 2013/July/13) Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 77.Shearer JC, Stack ML, Richmond MR, Bear AP, Hajjeh RA, Bishai DM. Accelerating policy decisions to adopt haemophilus influenzae type B vaccine: a global, multivariable analysis. PLoS medicine. 2010;7:1000249. doi: 10.1371/journal.pmed.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen A, Datta SD, Schwalbe N, Summers D, Adlide G. Working towards affordable pricing for HPV vaccines for developing countries: The role of GAVI. 2011 [Google Scholar]
- 79.Richter K. Implementation of HPV vaccination in South Africa. Public Health Association of South Africa. 2015 [Google Scholar]
- 80.De Vincenzo R, Conte C, Ricci C, Scambia G, Capelli G. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999–1010. doi: 10.2147/IJWH.S50365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Njeuhmeli E, Forsythe S, Reed J, Opuni M, Bollinger L, Heard L, et al. Voluntary Medical Male Circumcision: Modeling the Impact and Cost of Expanding Male Circumcision for HIV Prevention in Eastern and Southern Africa. PLoS medicine. 2011;8:e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rech D, Spyrelis A, Frade S, Perry L, Farrell M, Fertziger R, et al. Implications of the fast-evolving scale-up of adult voluntary medical male circumcision for quality of services in South Africa. PLoS One. 2014;9:e80577. doi: 10.1371/journal.pone.0080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohen MS, Baden LR. Preexposure prophylaxis for HIV--where do we go from here? N Engl J Med. 2012;367:459–461. doi: 10.1056/NEJMe1207438. [DOI] [PubMed] [Google Scholar]
- 84.Harris B, Goudge J, Ataguba JE, McIntyre D, Nxumalo N, Jikwana S, et al. Inequities in access to health care in South Africa. J Public Health Policy. 2011;32:102–123. doi: 10.1057/jphp.2011.35. [DOI] [PubMed] [Google Scholar]
- 85.Badri M, Maartens G, Mandalia S, Bekker LG, Penrod JR, Platt RW, et al. Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med. 2006;3:e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–24. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hankins C, Forsythe S, Njeuhmeli E. Voluntary medical male circumcision: an introduction to the cost, impact, and challenges of accelerated scaling up. PLoS Med. 2011;8:1001127. doi: 10.1371/journal.pmed.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venter F, Rees H, Pillay Y, Simelela N, Mbengashe TM, Geffen N, et al. The medical proof doesn't get much better than VMMC. S Afr Med J. 2012;102:124–125. doi: 10.7196/samj.5682. [DOI] [PubMed] [Google Scholar]
- 89.National Department of Health. HIV Counselling and Testing (HCT) Policy Guidelines. Pretoria, South Africa: National Department of Health; 2010. [Google Scholar]
- 90.Hedden L, O'Reilly S, Lohrisch C, Chia S, Speers C, Kovacic L, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist. 2012;17:164–171. doi: 10.1634/theoncologist.2011-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joint United Nations Programme on HIV/AIDS. MDG 6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS; 2015. How AIDS changed everything. [Google Scholar]
- 92.Harmon TM, Fisher KA, McGlynn MG, Stover J, Warren MJ, Teng Y, et al. Exploring the Potential Health Impact and Cost-Effectiveness of AIDS Vaccine within a Comprehensive HIV/AIDS Response in Low- and Middle-Income Cou…. PLoS One. 2016;11:146387. doi: 10.1371/journal.pone.0146387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galárraga O, Colchero MA, Wamai RG, Bertozzi SM. HIV prevention cost-effectiveness: a systematic review. BMC Public Health. 2009;9(Suppl 1):S5. doi: 10.1186/1471-2458-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stover J, Bollinger L, Hecht R, Williams C, Roca E. The impact of an AIDS vaccine in developing countries: a new model and initial results. Health Aff (Millwood) 2007;26:1147–1158. doi: 10.1377/hlthaff.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 95.Berndt ER, Glennerster R, Kremer MR, Lee J, Levine R, Weizsacker G, et al. Advance market commitments for vaccines against neglected diseases: estimating costs and effectiveness. Health Econ. 2007;16:491–511. doi: 10.1002/hec.1176. [DOI] [PubMed] [Google Scholar]
- 96.Joint United Nations Programme on HIV/AIDS. World AIDS Day 2015 on the Fast-Track to end AIDS by 2030 Focus on location and population. Geneva, Switzerland: 2015. [Google Scholar]
- 97.Raymond A, Hill A, Pozniak A. Large disparities in HIV treatment cascades between eight European and high-income countries -analysis of break points. J Int AIDS Soc. 2014;17:19507. doi: 10.7448/IAS.17.4.19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott HM, Klausner JD. Sexually transmitted infections and pre-exposure prophylaxis: challenges and opportunities among men who have sex with men in the US. AIDS Res Ther. 2016;13:5. doi: 10.1186/s12981-016-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keller SB, Smith DM. The price of tenofovir-emtricitabine undermines the cost-effectiveness and advancement of pre-exposure prophylaxis. AIDS. 2011;25:2308–2310. doi: 10.1097/QAD.0b013e32834d3cab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee DH, Vielemeyer O. Preexposure chemoprophylaxis for HIV prevention. N Engl J Med. 2011;364:1372–1373. doi: 10.1056/NEJMc1101504. author reply 1374-1375. [DOI] [PubMed] [Google Scholar]
- 101.Haire B. Considering Pre-Exposure Prophylaxis: Do the Pros Outweigh the Cons as an HIV Prevention Strategy? LGBT Health. 2014;1:253–255. doi: 10.1089/lgbt.2014.0081. [DOI] [PubMed] [Google Scholar]
- 102.Seidman D, Carlson K, Weber S, Witt J, Kelly PJ. United States family planning providers' knowledge of and attitudes towards pre-exposure prophylaxis for HIV prevention: A national survey. Contraception. 2016;95:463–469. doi: 10.1016/j.contraception.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 103.Knight R, Small W, Carson A, Shoveller J. Complex and Conflicting Social Norms: Implications for Implementation of Future HIV Pre-Exposure Prophylaxis (PrEP) Interventions in Vancouver, Canada. PloS one. 2016;11:146513. doi: 10.1371/journal.pone.0146513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koodibetse KG. HIV/AIDS stigma and discrimination in South Africa-still a problem. S Afr Med J. 2015;105:703. doi: 10.7196/samjnew.7811. [DOI] [PubMed] [Google Scholar]
- 105.Treves-Kagan S, Steward WT, Ntswane L, Haller R, Gilvydis JM, Gulati H, et al. Why increasing availability of ART is not enough: a rapid, community-based study on how HIV-related stigma impacts engagement to care in rural South Africa. BMC public health. 2016;16:2753–2762. doi: 10.1186/s12889-016-2753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gilbert L. ‘The mercurial piece of the puzzle’: Understanding stigma and HIV/AIDS in South Africa. SAHARA J. 2016;13:8–16. doi: 10.1080/17290376.2015.1130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toledo L, McLellan-Lemal E, Henderson FL, Kebaabetswe PM. Knowledge, Attitudes, and Experiences of HIV Pre-Exposure Prophylaxis (PrEP) Trial Participants in Botswana. World J AIDS. 2015;5:10–20. doi: 10.4236/wja.2015.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.White RG, Glynn JR, Orroth KK, Freeman EE, Bakker R, Weiss HA, et al. Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? AIDS. 2008;22:1841–1850. doi: 10.1097/QAD.0b013e32830e0137. [DOI] [PubMed] [Google Scholar]
- 109.Uthman OA, Popoola TA, Yahaya I, Uthman MM, Aremu O. The cost-utility analysis of adult male circumcision for prevention of heterosexual acquisition of HIV in men in sub-Saharan Africa: a probabilistic decision model. Value Health. 2011;14:70–79. doi: 10.1016/j.jval.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 110.Agot KE, Kiarie JN, Nguyen HQ, Odhiambo JO, Onyango TM, Weiss NS. Male circumcision in Siaya and Bondo Districts, Kenya: prospective cohort study to assess behavioral disinhibition following circumcision. J Acquir Immune Defic Syndr. 2007;44:66–70. doi: 10.1097/01.qai.0000242455.05274.20. [DOI] [PubMed] [Google Scholar]
- 111.Mattson CL, Campbell RT, Bailey RC, Agot K, Ndinya-Achola JO, Moses S. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PloS one. 2008;3:2443. doi: 10.1371/journal.pone.0002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrison A, Cleland J, Frohlich J. Young Peoples Sexual Partnerships in KwaZulu/Natal, South Africa: Patterns, Contextual Influences, and HIV Risk. Stud Fam Plann. 2008;39:295–308. doi: 10.1111/j.1728-4465.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mah TL, Maughan-Brown B. Social and cultural contexts of concurrency in a township in Cape Town, South Africa. Cult Health Sex. 2013;15:135–147. doi: 10.1080/13691058.2012.745951. [DOI] [PubMed] [Google Scholar]


