Figure 1. Comparison of mutational status of RAS/BRAF as determined by tumor tissue and plasma analysis at baseline (n=42).
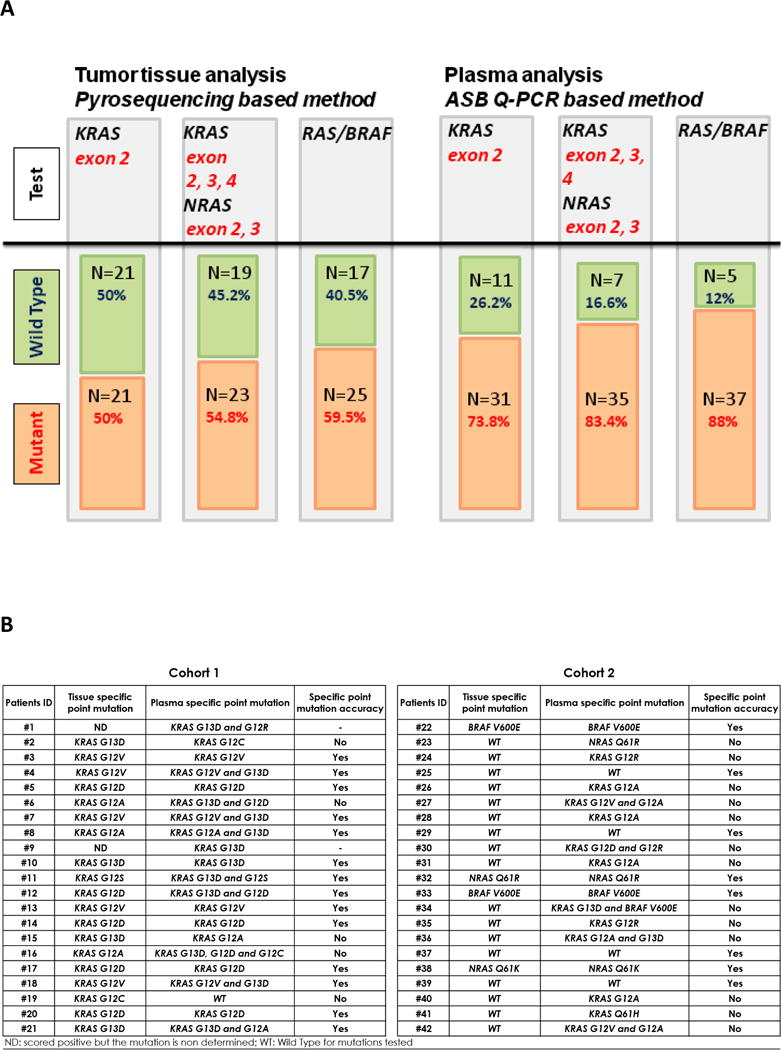
A) Plasma analysis was carried out in cohort 1 and 2 before initiation of treatments (baseline) according with targeted genes. B) Comparison of the type of point mutation as determined by tumor tissue and plasma analysis before initiation of treatments and for cohort 1 and 2. Extended RAS testing was only performed on plasma for patients who were KRAS exon 2 and BRAFV600E wild type on all assays. ND, scored positive but the mutation is non-determined; WT, wild type for mutations tested.
