Figure 3. MDH2 Acetylation and Canonical vs. 2-HG Generating Activity.
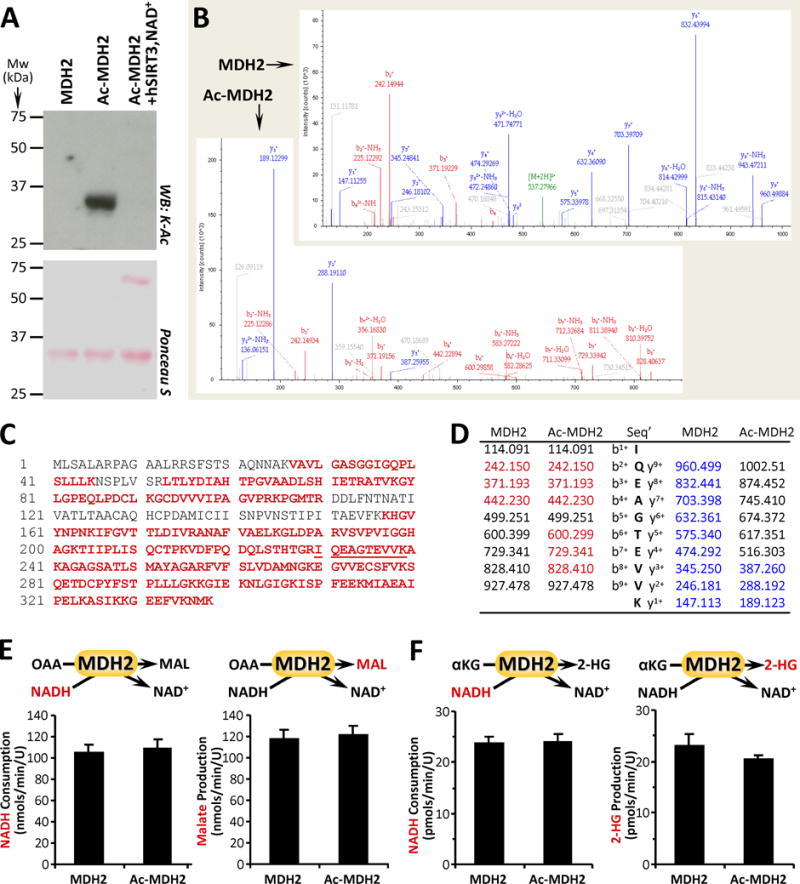
(A): Purified MDH2 was acetylated in-vitro with acetyl-CoA (see methods), followed by western blot probe with anti-acetyl-lysine (K-Ac) antibody. Ponceau S stained membrane below indicates protein loading. Images representative of at least 4 independent experiments. (B/C/D): Naïve and acetylated MDH2 (Ac-MDH2) were digested with trypsin and resultant peptides analyzed by mass spectrometry to identify acetylation sites. 76% sequence coverage was obtained (panel C, red font) including the peptide containing the proposed K239 acetylation site (underlined). Sample spectra are shown in B, with panel D listing identified peptides corresponding to bn+ ions (red) and yn+ ions (blue). A difference of 42 mass units between y+1 ion in MDH2 vs. Ac-MDH2 (189.123 – 147.113) indicates acetylation. (E): Canonical activity of naïve and acetylated MDH2 with OAA and NADH as substrates was measured spectrophotometrically as NADH consumption (left panel) or by LC-MS/MS as malate production (right panel). (F): Non-canonical activity of MDH2 with α-KG and NADH as substrates was measured spectrophotometrically as NADH consumption (left panel) or by LC-MS/MS as 2-HG production (right panel). All enzyme rate data are means ± SEM, n=4–6. Reactions monitored are shown above each graph, with the metabolite measured shown in red font.
