Table 3.
Studies of the Electronic Site Selectivity of the Aliphatic C–H Chlorination with N-Chloroamide 1.
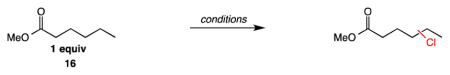
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | reagents | % selectivity of chlorination | |||||
| α | β | γ | δ | ω | |||
| MeO2C —CH2—CH2—CH2—CH2—CH3 | |||||||
| 1 | SO2Cl2, BPO, 85 °C | — | 12.1 | 37.7 | 42.0 | 8.2 | |
| 2 | Mn(TPP)Cl/NaOCl | — | 8.2 | 43.7 | 44.8 | 3.3 | |
| 3 | chloroamide 1, BPO, 65 °C | — | 10.8 | 37.9 | 42.4 | 8.9 | |
| 4 | chloroamide 1, hv, 1 equiv Cs2CO3, 55 °C | 3.6 | 4.6 | 19.7 | 57.6 | 14.4 | |
| (83% combined yield) | |||||||
|
| |||||||
| substrate (1 equiv) | % selectivity of chlorination | combined yield (%) | |||||
| α | β | γ | δ | ω | |||
| EWG —CH2—CH2—CH2—CH2—CH3 | |||||||
|
| |||||||
| 5 |
17 |
— | — | 4.9 | 81.2 | 13.9 | 80 |
| 6 |
18 |
— | — | 15.0 | 65.9 | 19.1 | 79 |
| 7 |
19 |
9.2 | 5.7 | 15.3 | 56.9 | 12.9 | 74 |
| 8 |
20 |
— | 9.0 | 19.8 | 57.3 | 13.8 | 89 |
| 9 |
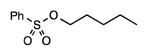 21 |
3.5 | 5.9 | 16.2 | 56.2 | 18.1 | 86 |
| 10 |
22 |
23.9 | 65.5 | 7.5 | 70 | ||
All reactions were performed with [substrate]0 = 1.0 M in PhH at rt under visible light irradiation with 1 equiv of substrate and 2 equiv chloroamide. Yields and selectivities determined by GC analysis.
