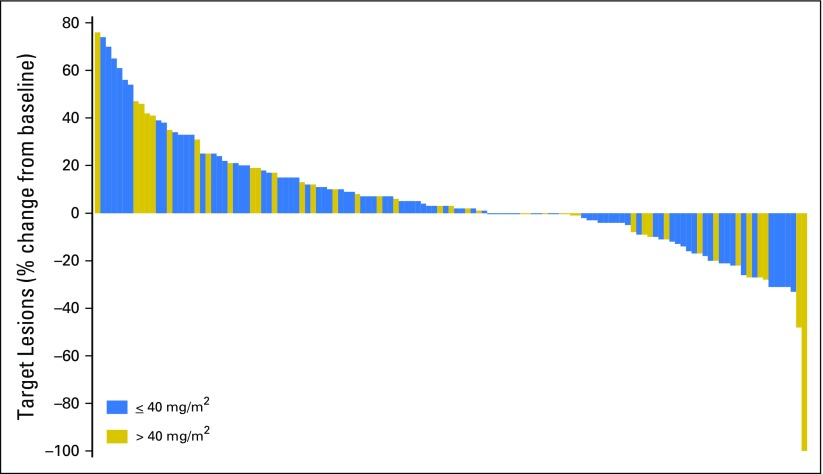
Fig 2.
Waterfall plot of change in target lesion volume after selinexor treatment. Best response in target lesions for 129 evaluable patients. Quantitative target lesion assessment was not available for the remaining 28 evaluable patients. These included 22 patients with progressive disease based solely on clinical symptoms and six patients with stable disease (SD), two of whom were patients with prostate cancer who had bone scans and remained on study for 325 and 72 days before showing disease progression. One patient with ovarian cancer had SD and remained on study for 365 days despite not having quantifiable baseline scans. The remaining three patients (two with prostate cancer and one with nasopharyngeal cancer) remained on study for 117, 71, and 44 days, respectively, without evidence of disease progression before withdrawing consent.