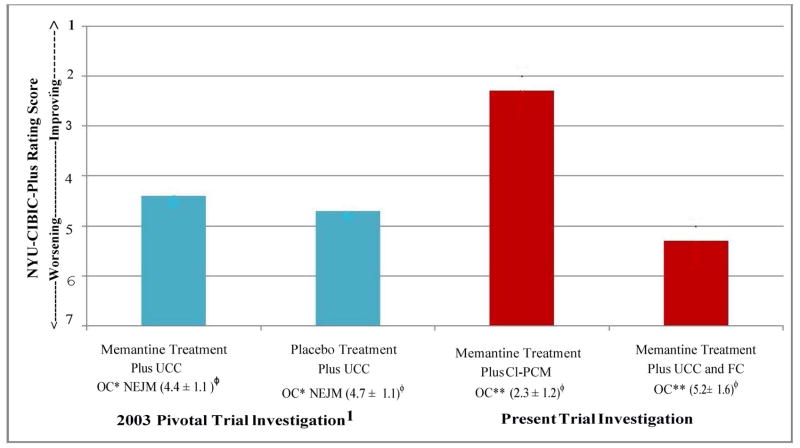
Figure 10. Comparison Between Results in the 2003 Memantine Pivotal Trial and the Current Results with the Comprehensive, Individualized, Person-Centered Management (CI-PCM) Program or Usual Community Care Plus Financial Compensation (UCC+FC) on the Primary Global Outcome Measurement, the New York University Clinician’s Interview-Based Impression of Change Plus Caregiver Input (NYU-CIBIC-Plus). All results are at the 28 Week Study Endpoint.
NYU-CIBIC-Plus, New York University Clinician’s Interview-Based Impression of Change-Plus Caregiver Input; UCC, Usual Community Care; CI-PCM, Comprehensive, Individualized, Person-Centered Management program; FC, Financial Compensation, up to $100 per subject; *OC, Observed Cases, i.e., completers of the 28-week pivotal trial1 or** of the 28-week trial in the present investigation in which one subject in the UCC+FC group was excluded from this analysis because of data quality concerns; NEJM, New England Journal of Medicine, published pivotal trial in 2003.1 Mean NYU-CIBIC-Plus score ± standard deviation, at each 28-week study endpoint is shown. For scoring details, see Figure 2 Legend. The current UCC+FC group might appear to be different from the 2003 pivotal trial memantine treatment and placebo treatment groups on the NYU-CIBIC-Plus assessment. However, the UCC+FC treatment group has a much smaller sample size. Therefore the differences in the UCC+FC group results in the present study, are not significantly different from treatment or the placebo group in the 2003 controlled trial on the NYU-CIBIC-Plus assessment.