Abstract
Purpose
We report pathologic, functional, and oncologic outcomes in patients treated with radical nephroureterectomy following radical cystectomy.
Materials and Methods
We identified patients who underwent radical cystectomy then radical nephroureterectomy for metachronous urothelial recurrence at our institution between January 1995 and December 2014. Univariable Cox regression was used to assess the association between overall survival and age, grade, stage, lymph node metastasis, and radiographic findings.
Results
Of the 3173 radical cystectomy patients, 64 underwent subsequent radical nephroureterectomy for metachronous urothelial recurrence. Median age at radical cystectomy was 66 years (IQR 61–74). Of the 64 who underwent radical nephroureterectomy, the median time from radical cystectomy to radical nephroureterectomy was 2.7 years (IQR: 1.4–4.6). Among 37 patients that underwent ureteroscopy prior to radical nephroureterectomy, 29 (78%) had positive biopsy. Radical nephroureterectomy pathology revealed 39% locally advanced disease (pT3/pT4) and 11% positive node status compared with 17% locally advanced disease, and 6% positive node status from radical cystectomy pathology. Post- radical nephroureterectomy eGFR was <60 mL/min/1.73m2 and <30 mL/min/1.73m2 for 96% and 40% of patients, respectively. Median overall survival from radical nephroureterectomy was 3.1 years (95% CI: 2.4–4.3). Only lymph node involvement on radical nephroureterectomy was significantly associated with worse overall mortality (HR=2.73, 95% CI 1.04, 7.15, p=0.041).
Conclusions
Prognosis is poor for patients with panurothelial carcinoma treated with nephroureterectomy following cystectomy, with locally advanced disease in a large proportion of patients. Renal function after these procedures diminished and almost all patients were ineligible for cisplatin-based chemotherapy.
Keywords: cystectomy, nephroureterectomy, upper tract urothelial carcinoma, urinary bladder neoplasms, treatment outcome
Introduction
Urothelial carcinoma may arise from a “panurothelial” field defect in the entire epithelial lining of the urinary tract, which is characterized by frequent, multifocal metachronous tumors. The biological behavior and treatment approaches of urothelial tumors are extremely heterogeneous. Radical cystectomy (RC) is the gold standard of treatment for patients with muscle-invasive UCB and for patients with noninvasive UCB that is refractory to intravesical therapy. Compared with UCB, Upper tract urothelial carcinoma (UTUC) is a rare disease, accounting for approximately 5–10% of all urothelial carcinomas.1 Radical nephroureterectomy (RNU) with excision of the bladder cuff is the gold standard of treatment for organ-confined disease. Previous studies only included 16–24 patients with upper tract recurrence following radical cystectomy.2, 3 There is little information regarding clinic management and outcomes in patients with panurothelial carcinoma disease. Metachronous involvement of the upper tract following RC represents a type of aggressive panurothelial disease that may warrant specialized management strategies. We report pathologic, renal functional and oncologic outcomes in patients who underwent a radical nephroureterectomy to treat metachronous upper tract recurrence following cystectomy at a tertiary referral center.
Materials and Methods
After institutional review board approval, we retrospectively reviewed prospectively collected data on patients who underwent RC and urinary diversion for UCB at Memorial Sloan Kettering Cancer Center from January 1995 through December 2014. Of the 3173 patients who underwent RC, we identified 64 patients who underwent subsequent RNU for metachronous upper tract recurrence for final analysis. We excluded patients who had prior history of UTUC, concurrent UTUC, concurrent RNU, and subsequent RNU for nonfunctional kidney secondary to ureteral stricture. Demographic, clinical, and pathological data were collected by chart review.
After RC, patients were generally followed every 3 months in year 1, every 4 months in year 2, every 6 months in years 2–5, and annually thereafter. Urine cytology was obtained at each visit. Baseline upper tract imaging was obtained in most patients after cystectomy followed by annual imaging and imaging obtained as clinically indicated for evaluation of hematuria, systemic symptoms, or abnormal cytology findings. The indications for RNU included endoscopically or pathologically proven recurrence, positive selective cytology, and/or abnormal radiographic findings of recurrence in the renal collecting system or ureter. All upper tract recurrences were pathologically confirmed by cytology, or endoscopic biopsy (antegrade or retrograde ureteroscope), or final RNU pathology. eGFR was calculated using the abbreviated Modification of Diet in Renal Disease equation, eGFR=186x (serum creatinine)-1.154 × (age)-0.203 multiplied by 0.742 male patients and 1.212 for black patients.4 Postoperative eGFR was measured close to 6 months (range 3–12 months) following RNU and compared with eGFR before RC and RNU.
OS was determined from the date of RNU until death or until the most recent patient contact. Univariable Cox proportional hazards regression was used to test the association between survival and the following factors: RC and RNU grade (low grade versus high grade), RC and RNU stage (≤pT2 versus pT3/pT4), RNU surgical margin, RNU lymph node metastasis, pre-RC CKD-EPI estimated eGFR, pre-RNU CKD-EPI estimated eGFR, and various radiographic criteria including hydronephrosis, filling defect, wall thickening, mass lesions and enlarged lymph nodes. All analyses were conducted using Stata 13 (Stata Corp., College Station, TX).
Results
Table 1 displays the pathological features of RC and RNU. The median age at RC was 66 (IQR 61–74). Among the 64 patients we identified who underwent subsequent RNU, the median time from RC and RNU was 2.7 years (IQR 1.4–4.6). RNU pathology revealed that 39% of patients had locally advanced disease (pT3/pT4) while 17% of patients had locally advanced disease at the time of RC. Similarly, lymph node metastases and soft tissue margins were more commonly found at the time of RNU than at the time of RC (13% versus 6% and 14% versus 2%, respectively). Cytology prior to RNU was positive for malignancy in 37 (58%) patients. Of 37 patients undergoing endoscopy prior to RNU, 29 (78%) had positive biopsies. Among 31 patients who underwent RNU without previous positive or suspicious biopsy, 1 patient with a poorly functioning kidney and suspicious ureteral wall thickening was pT0. The majority of patients with upper-tract disease were detected by imaging prior to RNU. The most common radiographic findings were irregular wall thickening/enhancement in 31 (48%) patients, followed by mass lesions in 24 (38%), hydronephrosis in 18 (28%), and filling defect in 9 (14%) (Table 2).
Table 1.
Characteristics and pathologic features of patients undergoing radical cystectomy (RC) and radical nephroureterectomy (RNU). Data are median (quartiles) or frequency (percentage).
| Patients | n=64 |
|---|---|
| Age at RC | 66 (61–74) |
| Time from RC to RNU years median (interquartile range) | 2.7 (1.4–4.6) |
| <2 years | 24 (38%) |
| 2–5 years | 27 (42%) |
| >5 years | 13 (20%) |
| Male | 52 (81%) |
| Smoker | 50 (78%) |
| Race | |
| Other | 3 (4.7%) |
| White | 61 (95%) |
| Pathological features on RC | |
| Ureteral Involvement | 27 (42%) |
| Concomitant CIS | 46 (72%) |
| Pathological T Stage on RC | |
| <=pT2 | 53 (83%) |
| pT3/pT4 | 11 (17%) |
| Pathological Grade on RC | |
| Low | 7 (11%) |
| High | 46 (72%) |
| Unknown | 11 (17%) |
| Margin Status on RC | |
| Negative | 62 (97%) |
| Positive | 1 (1.6%) |
| Unknown | 1 (1.6%) |
| Lymph Node Involvement on RC | |
| N0 | 56 (88%) |
| N1 | 4 (6.3%) |
| NX | 4 (6.3%) |
| Pathological features on RNU | |
| Tumor Location | |
| Renal Pelvis | 20 (31%) |
| Ureter | 12 (19%) |
| Renal Pelvis + Ureter | 29 (45%) |
| T0 | 3 (4.7%) |
| Pathological T Stage on RNU | |
| <=pT2 | 39 (61%) |
| pT3/pT4 | 25 (39%) |
| Pathological Grade on RNU | |
| Low | 3 (4.7%) |
| High | 52 (81%) |
| Unknown | 9 (14%) |
| Margin Status on RNU | |
| Negative | 51 (80%) |
| Positive | 9 (14%) |
| Unknown | 4 (6.3%) |
| Lymph Node Involvement on RNU | |
| N0 | 32 (50%) |
| Z | 8 (13%) |
| NX | 24 (38%) |
Table 2.
Preoperative findings before radical nephroureterectomy (RNU).
| Patients | n=64 |
|---|---|
| Pre-RNU cytology | |
| Negative | 19 (30%) |
| Positive | 37 (58%) |
| Suspicious/atypical | 7 (11%) |
| Unknown | 1 (1.6%) |
| Pre-RNU ureteroscopy | |
| Upper tract biopsy result (n=35) | 35 (55%) |
| Negative | 1 (2.9%) |
| Positive | 27 (77%) |
| Suspicious/atypical | 6 (17%) |
| Unknown | 1 (2.9%) |
| Pre-RNU abnormal radiographic findings | |
| Hydronephrosis | 18 (28%) |
| Filling defect | 9 (14%) |
| Irregular wall thickening/enhancing | 31 (48%) |
| Mass lesions | 24 (38%) |
| Enlarged lymph node | 4 (6.3%) |
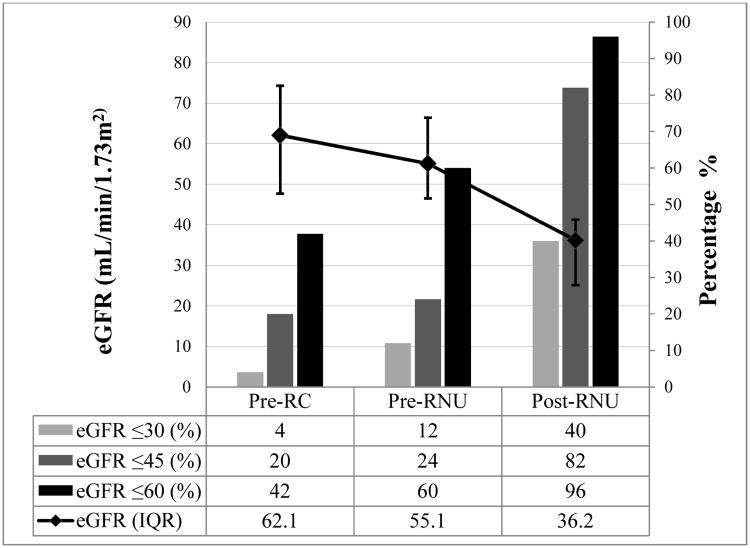
Renal function outcomes are summarized in Figure 1. Among the 50 patients with complete eGFR measurements, median eGFR (55 mL/min/1.73m2) declined slightly from baseline eGFR (62 mL/min/1.73m2) after RC, whereas median eGFR decreased substantially (36 mL/min/1.73m2) after RNU. Following RNU, 48 patients (96%) had eGFR ≤60 mL/min/1.73m2 and 20 patients (40%) had eGFR ≤30 mL/min/1.73m2.
Figure 1.
Impact of radical cystectomy (RC) and radical nephroureterectomy (RNU) on renal function among those who had complete data and a minimum 1-year follow-up following RNU (n=50). Post-RNU was measured closest to 6 months (range 3–12). The progressive decline of median (interquartile range [IQR]) estimated glomerular filtration rates (eGFR) after RC and RNU are shown on the left axis. The percentages of patients with eGFR ≤ 30 mL/min/1.73m2, eGFR ≤ 45 mL/min/1.73m2, and eGFR ≤ 60 mL/min/1.73m2 are shown on the right axis.
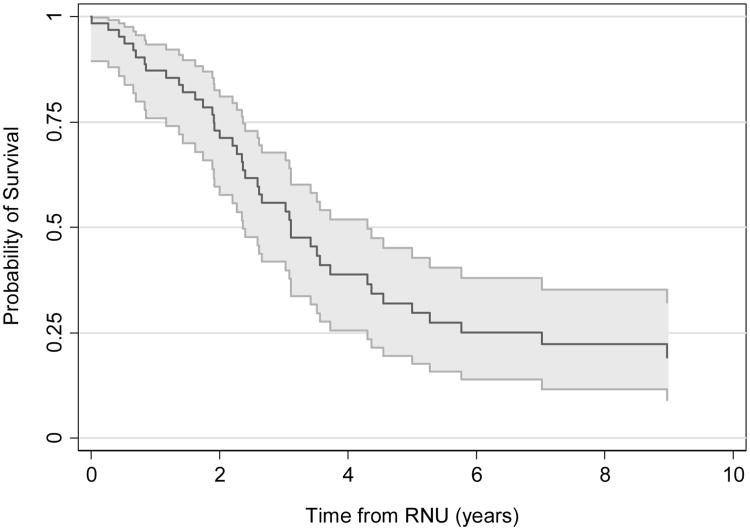
Eleven patients (17%) received chemotherapy prior to diagnosis of UTUC, including neoadjuvant (6), or adjuvant (3), or salvage (1) chemotherapy for UCB, or chemotherapy other disease (1). Among 16 patients undergoing neoadjuvant gemcitabine/cisplatin prior to RNU, 9 (56%) died and the remaining 7 were alive at a median followup of 2.0 years. Thirteen patients received chemotherapy after RNU, but none of the regimens (taxol, gemcitabine, carboplatin, or clinical trials) were cisplatin-based owing to poor renal function. All 13 patients who received chemotherapy after RNU subsequently died. Median follow-up time for the entire cohort was 2.9 years (IQR 1.6–6.5) for survivors. The 5-year estimated probability of OS was 32% (95% CI 20%, 45%). Median OS was 3.1 years (95% CI: 2.4–4.3; Figure 2). We found that among all clinical or pathological features of interest, only lymph node involvement was significantly associated with a higher risk of overall mortality (HR=2.73, 95% CI 1.04, 7.15, p=0.041; all other p-values ≥0.15; Supplement Table 1).
Figure 2.
Probability of overall survival after radical nephroureterectomy (RNU).
Discussion
Patients who undergo RC for urothelial carcinoma have a lifelong-risk of upper tract disease in the remnant urothelium. Guidelines refer to surveillance after upper tract or lower tract extirpative surgeries, but do not address the management of panurothelial disease.5, 6 In this study, we found subsequent worse pathologic features in the upper tract compared with the pathologic features in the bladder in patients treated with RNU following RC. These patients have poor OS, and progressively diminished renal function, which rendered almost all patients ineligible for cisplatin-based adjuvant chemotherapy after sequential extirpative treatment. We found lymph node metastasis at the time of RNU was the only prognostic factor associated with overall mortality.
The greatest risk factor for the development of UTUC is a previous history of UCB. A recent meta-analysis of 13,185 patients found that the overall prevalence of upper tract recurrence after cystectomy was 0.75% to 6.4% at a range of 2.4 to 164 months.7 More importantly, the meta-analysis demonstrated that patients with a low-grade tumor, concurrent CIS lesion, multifocal lesions, superficial cancer, positive ureteral/urethral margin and negative nodal status were more likely to develop upper tract recurrence based on the cystectomy specimen. Similarly, our cohort had relatively favorable pathologic features in RC specimen of 87% organ-confined disease (<pT2), and 6% positive node status compared with the overall incidence of 40-45% extravesical disease (pT3/pT4) and 20-25% positive node status in large retrospective cystectomy studies.8, 9 In contrast, when RNU was performed for metachronous upper tract recurrence, patients demonstrated unfavorable pathologic features with 39% having locally advanced disease and 11% having positive node status. The disparity between RNU pathology and RC pathology may be explained by the fact that patients with unfavorable pathologic features in the RC specimen may have died from the disease before experiencing metachronous upper tract recurrence. The alternative plausible biological explanation may be related to the accumulated mutations in metachronous UTUC compared with the genetic alterations from UCB at initial presentation. We found the median time from RC to RNU was 2.7 years and also observed 20% of late UTUC recurrence 5 years after cystectomy. We are currently investigating the different genetic alterations between UCB and subsequent UTUC in the same patients with late urothelial recurrence. This may further delineate the sequential genetic hits of driver mutations in patients with panurothelial disease. European Association of Urology Guidelines state that a previous history of RC is a high-risk factor for risk stratification of UTUC and these patients may be considered for extirpative treatment.5, 10 The poor prognosis in our study suggests that specialized multimodality strategies should be considered, which may improve the oncologic outcomes in this highly vulnerable group of patients.
Guidelines have provided surveillance protocols of cytology, imaging studies, and urethral wash for UCB following RC. In accordance with surveillance guidelines, we found that cytology was positive in 58% of all patients with pathologically confirmed upper tract recurrence. In contrast, all patients had at least one abnormal radiographic finding, most commonly demonstrating wall thickening/enhancement/irregularity followed by mass lesions and hydronephrosis. Based on the physician's discretion, antegrade or retrograde endoscopic biopsies were performed in 55% of patients with 77% of the biopsies positive for malignancy. With the exception of 1 patient, all who underwent RNU on the basis of radiographic evidence alone were found to harbor urothelial cancers. This is consistent with a recently proposed “empiric evidence-based algorithm” for UTUC in which ureteroscopy can be omitted as part of the diagnostic workup in appropriately selected patients planning to undergo extirpation of UTUC based on abnormal radiographic and cytologic findings.11
Decline in renal function has been described in patients with UCB and UTUC after radical surgery. A recent study demonstrated decreased renal function during long-term follow-up in most patients who underwent RC, demonstrating a decrease from a median of 62 mL/min/1.73m2 at baseline to a median of 55 mL/min/1.73m2 at 5 years, and a median of 51 mL/min/1.73m2 at 10 years.12 In contrast, the median eGFR decreased from 60 mL/min/1.73m2 to 47 mL/min/1.73m2 after RNU, resulting in only 19% of patients eligible for chemotherapy.13 We found that patients had progressively diminished renal function following RC and RNU due to the deteriorating effects of these extirpative surgeries, with renal function in 40% of patients deteriorating to stage 4 chronic kidney diseases in our cohort. More importantly, using a cut-off of 60 mL/min/1.73m2, 60% of patients were ineligible for neoadjuvant chemotherapy before RNU, and 96% of patients were ineligible for adjuvant chemotherapy after RNU.6, 13 A recent meta-analysis suggested that cisplatin-based chemotherapy for UTUC in the neoadjuvant setting had promising survival benefit (pooled hazard ratio (HR) for OS was 0.43, p = 0.023) compared with those who received surgery alone.14 Therefore, neoadjuvant therapeutic strategies should be strongly considered in patients with aggressive features when possible. We are currently enrolling all eligible high-risk patients with UTUC in a clinical trial of neoadjuvant chemotherapy with gemcitabine and cisplatin (NCT01261728); other related cooperative group trials are also accruing (NCT02412670). Renal function compromise not only limits therapeutic options but may also contribute to other-cause mortality noting that reduced eGFR has been independently associated with risk of death and cardiovascular events.15 However, due to variability in eGFR measurements, statistical modeling constraints and other confounding factors the impact of postoperative eGFR on survival was difficult to assess. Noting the many complexities, management of metachronous UTUC after cystectomy should be individualized by multidisciplinary teams, who take into account multiple factors, such as cancer control, the need for dialysis/kidney transplantation, and options for further systemic therapy.
It is controversial whether UTUC and UCB have different oncologic outcomes. Rink et al. found stage-specific different oncologic outcomes between UTUC and UCB following radical surgery,9 whereas others reported comparable oncologic outcomes.16 Nevertheless, studies showed that OS from the time of upper tract recurrence after RC was poor, and median OS was 0.8 to 4.6 years with most patients dying of metastatic disease.2, 3, 17 Favaretto et al. previously reported the 5-year CSS of 78% in patients with primary UTUC at our institution, excluding patients with previous or concurrent RC.18 The 5-year probability of OS in our group was 32% (95% CI 20%, 45%), suggesting more aggressive behavior of panurothelial disease than that of primary UTUC. In addition, we identified lymph nodal metastasis as a prognostic factor associated with overall mortality, while other authors found that pathological tumor stage, lymphvascular invasion, tumor architecture and nodal status were independently associated cancer-specific survival in patients with primary UTUC.18, 19 It also should be noted that the topic of panurethelial disease in patients with metachronous intravesical recurrence after primary RNU was not addressed in this study, since it may represent different natural history and biology given the disparity of recurrence patterns and oncologic outcomes. Tanaka et al. demonstrated that 40% of patients experienced intravesical recurrence (70% pTa and 30% pT1) following RNU for primary UTUC and bladder progression (muscle invasion or greater) was only 5% after intravesical bacillus Calmette–Guérin (BCG) or intravesical chemotherapy.20 Kim et al. reported a similar finding of 41% intravesical recurrence and 6% muscle invasive UCB in 422 patients who underwent RNU for primary UTUC after a median follow-up of 44 months.21
The first limitation of this retrospective study is the inherent selection and referral bias. Only patients with aggressive disease that warrant both RC and subsequent RNU in a single high-volume tertiary cancer center were included. This selection possibly underestimated OS of patients with metachronous upper tract recurrence because patients amenable to radical surgery may have significantly better survival than those who are unable to undergo radical surgery.2, 22 Second, our study was limited by the variability of the timing of postoperative serum eGFR measurements.13 Third, the lack of other compounding factors, such as history of smoking and exposure to carcinogens, may overlook the risk of panurothelial tumorigenesis. Despite these limitations, our study represents the largest series of patients with panurothelial disease managed surgically and includes additional insight into the pathologic features and clinical outcomes of this poor-risk group.
Conclusions
Current management of metachronous urothelial recurrence after cystectomy is clinically challenging with limited evidence for informed clinical decision making. We have demonstrated poor prognosis in patients with panurothelial disease treated with RNU following cystectomy, especially in patients with nodal involvement. Neoadjuvant therapeutic strategies should be strongly considered in high-risk patients prior to nephroureterectomy when possible due to the medically compromised state of patients in this setting. Given the variability in disease behavior and the diversity of treatment options, better risk stratification strategies and novel treatment techniques are necessary to avoid unnecessary RNU and optimize kidney-sparing approaches. In the era of precision medicine, emerging biomarkers of risk, predicting therapeutic response, novel topical agents, and multi-modal therapies could serve to optimize care for these highly vulnerable patients.
Supplementary Material
Supplementary Table 1. Univariate Cox proportional hazards regression to determine factors associated with overall survival after radical nephroureterectomy.
Acknowledgments
We thank Joyce Tsoi for editorial comments.
Funding: Supported in part by funds from David H. Koch provided through the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers, T32 CA082088 (Qiang Li, MD; PI: Brett S. Carver, MD), and P30 CA008748 (PI: Craig B. Thompson, MD).
Abbreviations and Acronyms
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
estimated glomerular filtration rate
- OS
overall survival
- RC
radical cystectomy
- RNU
radical nephroureterectomy
- UCB
urothelial carcinoma of the bladder
- UTUC
upper tract urothelial carcinoma
Footnotes
Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Roupret M, Babjuk M, Comperat E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Balaji KC, McGuire M, Grotas J, et al. Upper tract recurrences following radical cystectomy: an analysis of prognostic factors, recurrence pattern and stage at presentation. J Urol. 1999;162:1603. doi: 10.1016/s0022-5347(05)68176-1. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Alemozaffar M, Harris BN, et al. Outcomes after urothelial recurrence in bladder cancer patients undergoing radical cystectomy. Urology. 2014;84:1420. doi: 10.1016/j.urology.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 4.Silberstein JL, Power NE, Savage C, et al. Renal function and oncologic outcomes of parenchymal sparing ureteral resection versus radical nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2012;187:429. doi: 10.1016/j.juro.2011.09.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roupret M, Babjuk M, Comperat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur Urol. 2015;68:868. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Picozzi S, Ricci C, Gaeta M, et al. Upper urinary tract recurrence following radical cystectomy for bladder cancer: a meta-analysis on 13,185 patients. J Urol. 2012;188:2046. doi: 10.1016/j.juro.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol. 2007;178:2308. doi: 10.1016/j.juro.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Rink M, Ehdaie B, Cha EK, et al. Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62:677. doi: 10.1016/j.eururo.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Potretzke AM, Knight BA, Potretzke TA, et al. Is Ureteroscopy Needed Prior to Nephroureterectomy? An Evidence-Based Algorithmic Approach. Urology. 2016;88:43. doi: 10.1016/j.urology.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg MS, Thompson RH, Frank I, et al. Long-term renal function outcomes after radical cystectomy. J Urol. 2014;191:619. doi: 10.1016/j.juro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Kaag MG, O'Malley RL, O'Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58:581. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leow JJ, Martin-Doyle W, Fay AP, et al. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66:529. doi: 10.1016/j.eururo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 16.Moussa S, Yafi FA, El-Hakim A, et al. Outcome of surgical treatment of patients with upper versus lower urinary tract urothelial carcinoma: stage-by-stage comparison. Urol Int. 2010;84:50. doi: 10.1159/000273466. [DOI] [PubMed] [Google Scholar]
- 17.Soukup V, Babjuk M, Bellmunt J, et al. Follow-up after surgical treatment of bladder cancer: a critical analysis of the literature. Eur Urol. 2012;62:290. doi: 10.1016/j.eururo.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Favaretto RL, Shariat SF, Chade DC, et al. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol. 2010;58:574. doi: 10.1016/j.eururo.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2012;61:818. doi: 10.1016/j.eururo.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka N, Kikuchi E, Kanao K, et al. Independent predictors for bladder outcomes after treatment of intravesical recurrence following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma. Ann Surg Oncol. 2014;21:3151. doi: 10.1245/s10434-014-3657-y. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, You D, Jeong IG, et al. Muscle-invasive bladder cancer developing after nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1643. doi: 10.1016/j.urolonc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Raj GV, Bochner BH, Serio AM, et al. Natural history of positive urinary cytology after radical cystectomy. J Urol. 2006;176:2000. doi: 10.1016/j.juro.2006.07.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Univariate Cox proportional hazards regression to determine factors associated with overall survival after radical nephroureterectomy.