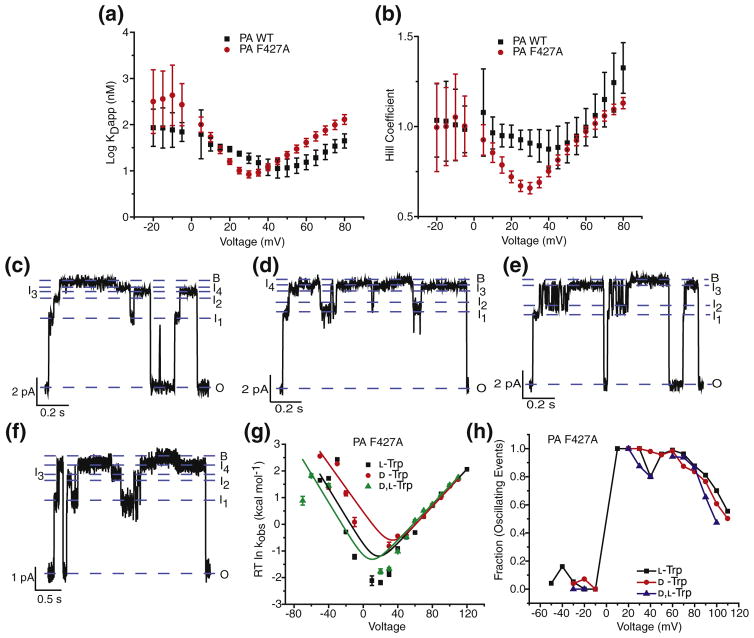
Fig. 5.
ϕ clamp mutation alters translocation mechanism. (a) The dissociation constants (log KDapp) from the ensemble steady-state measurements of L-Trp through WT PA channels (black) and PA F427A channels (red) plotted against voltage. (b) Plot of Hill coefficients of L-Trp through WT PA channels (black) and PA F427A channels (red) against voltage. Single-channel planar lipid bilayer current records at symmetric pH 5.6 and 100 mM KCl of PA F427A, following cis-side addition of (c) L-Trp (d) D-Trp and (e) D,L-Trp peptides at 70 mV, and (f) L-Trp at 50 mV. Open (O), blocked (B), and intermediate (I1 – I4) states are indicated. Records are Gaussian-filtered to 50 Hz. (g) Chevron plots of the observed rate constants (kobs) for the blocked-state dwell times of PA F427A in the presence of L-Trp (black), D-Trp (red), and D,L-Trp (green) peptides, plotted as ΔG‡ = RT ln kobs as a function of voltage. The data are fit to the chevron function defined in Materials and Methods. (h) Plot of fraction of events with subconductance state(s) (oscillating events), against voltage (mV), in PA F427A channel in the presence of L-Trp (black), D-Trp (red), and D,L-Trp (blue) peptides.