Figure 2.
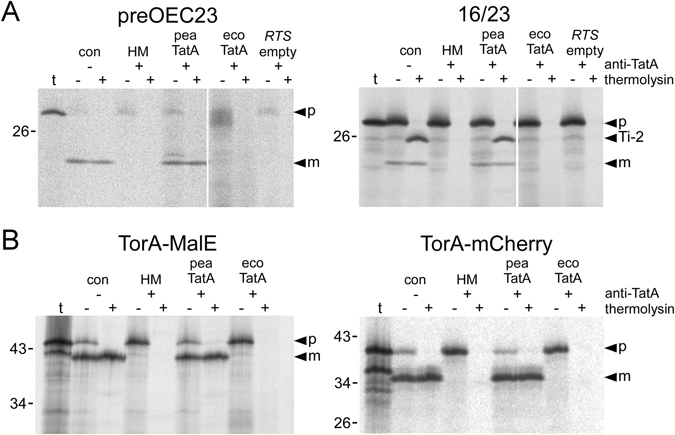
Reconstitution of thylakoidal Tat transport with TatA from pea and E. coli. (A) In thylakoido transport of authentic and chimeric plant model Tat substrates in the presence of TatA from pea or E. coli. The authentic precursor of OEC23 (preOEC23) and the chimeric precursor protein 16/23 were generated by in vitro transcription of the respective cDNA clones and subsequent in vitro translation in the presence of [35S]-methionine. 5 μl of each translation assay were incubated with thylakoid vesicles isolated from pea that had been either mock-treated (con) or treated with antibodies against TatA (+anti-TatA). The latter were subsequently supplemented with either HM buffer only (10 mM Hepes/KOH, pH 8.0; 5 mM MgCl2) (HM) or with TatA from pea (pea TatA) or E. coli (eco TatA), which were obtained by in vitro transcription/translation with the wheat germ rapid translation system (RTS). Lanes RTS empty show translation assays containing an empty vector control. After incubation for 15 min in the light at 25 °C, thylakoids were washed once with HM buffer and subsequently incubated with either thermolysin (182 μg/ml, 30 min on ice, lanes+) or HM buffer (lanes−). From each fraction stoichiometric amounts corresponding to 7.5 μg of chlorophyll were analysed on 10–17.5% SDS-polyacrylamide gradient gels and detected by phosphorimaging. In lanes t, 1 μl of the translation assays of the respective Tat substrates was loaded. The bands showing the precursor (p) and mature proteins (m) are indicated by filled arrowheads. Ti-2 marks the protease-protected fragment indicative of translocation intermediate Ti-2 of the 16/23 chimera in which the passenger protein is fully translocated across the membrane but the Tat transport signal not yet removed24, 48. The molecular weights (in kDa) of marker proteins loaded in parallel are indicated on the left of each panel. (B) In thylakoido transport of the bacterial model Tat substrates TorA-MalE and TorA-mCherry.
