Figure 4.
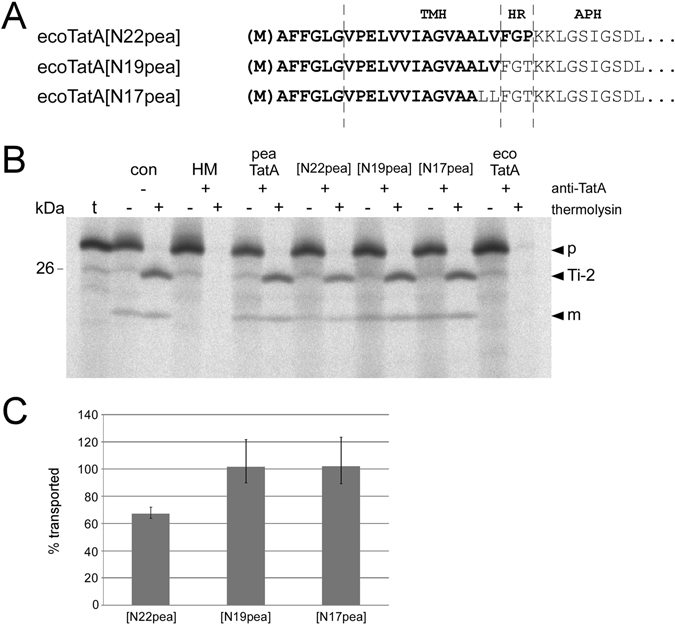
Catalytic activity of chimeric TatA proteins in in thylakoido complementation experiments. (A) Amino acid sequence of the N-terminal regions of the chimeric TatA proteins ecoTatA[N22pea], ecoTatA[N19pea], and ecoTatA[N17pea]. The residues derived from pea are depicted in bold. Please note that due to the use of E. coli TatA as reference the number of pea residues in each chimera is actually two residues higher than depicted in the name (see also Fig. 1). (B) I n thylakoido complementation of 16/23 transport by the RTS-generated chimeric TatA proteins shown in (A). For further details see the legends to Figs 1 and 2. (C) Quantitation of catalytic activities as shown in (B) given as percentage of the transport rate determined for the antibody-treated assays supplemented with pea TatA. Both mean values and standard deviations from three (n = 3) independently repeated experiments are shown.
