Fig. 1.
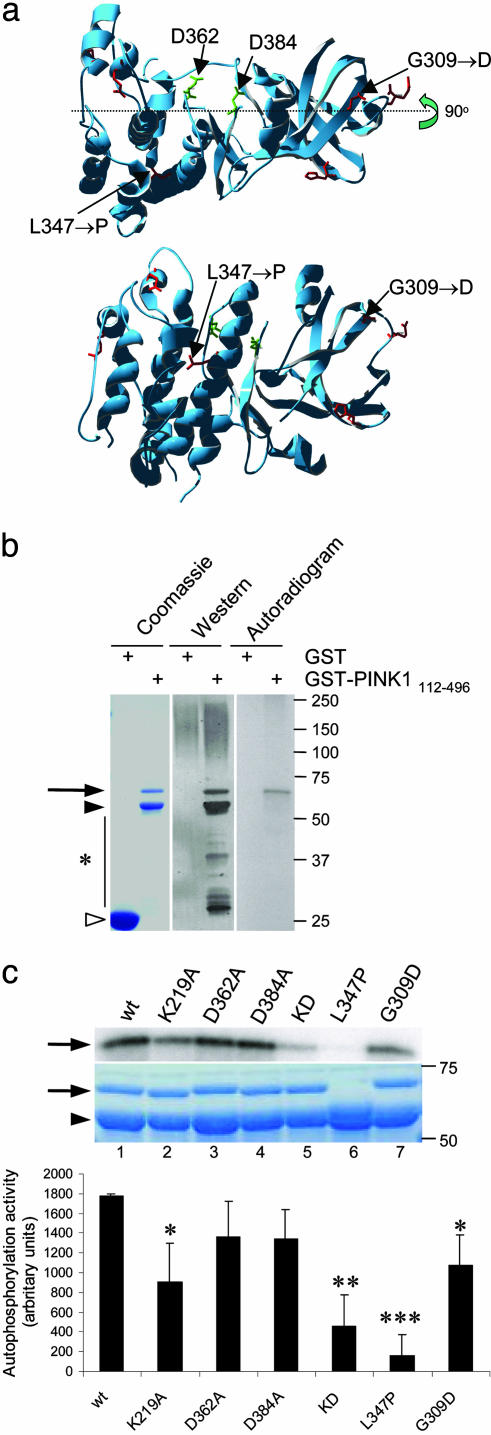
Kinase activity of PINK1 and the effects of mutations in vitro.(a) Model of kinase domain of PINK1 showing positions of critical aspartates and the two recessive mutations investigated in this study. Lower is rotated by 90° at the indicated axis. (b) Expression and autophosphorylation activity of wild-type PINK1 GST-fusion proteins showing, from left to right, Coomassie-stained SDS/PAGE gel, Western blot, and autoradiogram. Alternate lanes contain GST alone, as indicated. Arrow indicates GST-PINK1 fusion proteins, filled arrowhead shows prominent breakdown product, and open arrowhead shows GST alone. Markers on the right of all gels or blots are in kilodaltons. (c) Kinase activity of PINK1 mutants showing autoradiogram (Top), Coomassie staining (Middle), and quantitation of incorporated radioactivity, corrected for protein loading. Wild-type PINK1 (lane 1) is shown for comparison with artificial kinase mutants (K219A, lane 2; D362A, lane 3; D384A, lane 4; triple kinase-dead mutant, lane 5). Two recessive mutants associated with human parkinsonism, L347P and G309D, are shown in lanes 6 and 7. (Bottom) Bars show the average from three independent experiments, with error bars showing SEM. Differences in activity between variants were assessed by ANOVA with Fisher's PLSD post hoc test; *, P < 0.05; **, P < 0.01; ***, P < 0.001.