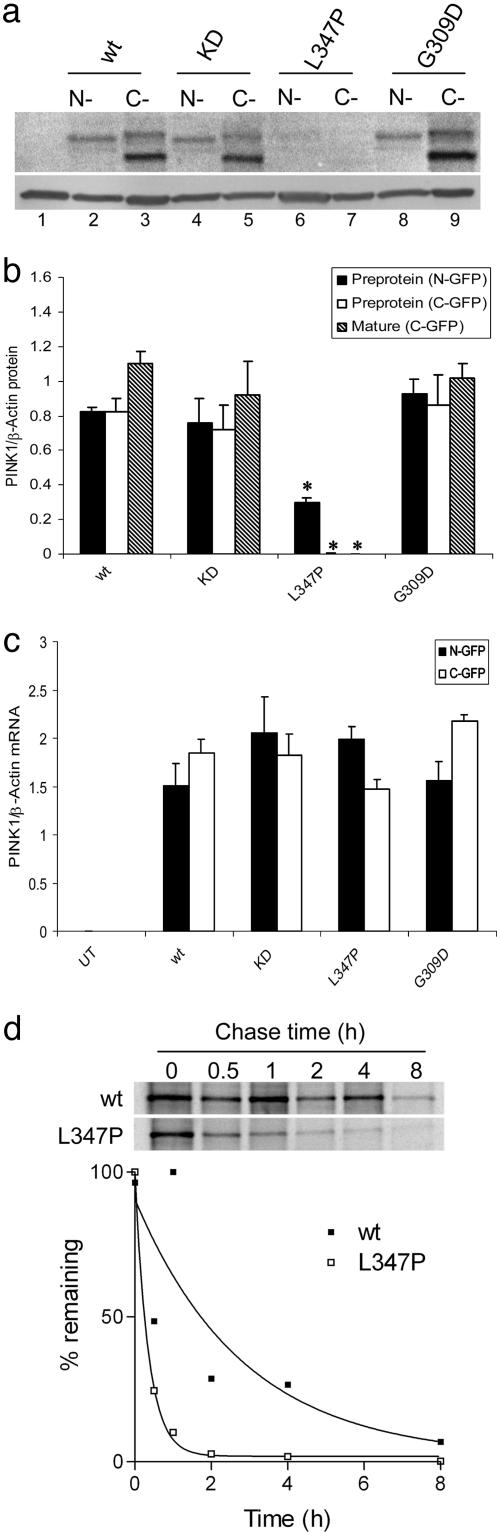
Fig. 4.
Effects of mutations on PINK1 protein stability in mammalian cells. (a) COS-7 cells were transfected with N-terminal (lanes 2,4,6 and 8) or C-terminal (lanes 3, 5, 7, and 9) GFP-tagged PINK1 variants or left untransfected as a control (lane 1). Steady-state protein levels were decreased for L347P (lanes 6 and 7) but not G309D (lanes 8 and 9) recessive mutant PINK1 compared with wild type (lanes 2 and 3) control or the artificial kinase-dead construct (lanes 4 and 5). The arrow indicates the position of the preprotein, the filled arrowhead shows the mature protein, and the open arrowhead shows the position of β-actin used as a loading control. We quantified both protein (b) and mRNA (c) expression for all constructs from n = 3 experiments. For protein data, we measured the intensity of the preprotein band from the N-terminal construct (filled bars) with both the preprotein (open bars) and mature protein (hatched bars) from the C-terminal construct. *, protein levels were significantly different from wild-type construct (P < 0.01 by ANOVA). (d) Pulse–chase analysis of protein stability. COS-7 cells were transiently transfected with N-terminal GFP-tagged wild-type (Upper) or L347P (Lower) PINK1, labeled with 35S-Met/Cys and chased with unlabeled media for 0–8 h as indicated above each lane. Quantification of this experiment is shown, with curves fitted to a single-phase exponential decay function. Similar estimates of stability were obtained in duplicate experiments.