Figure 2.
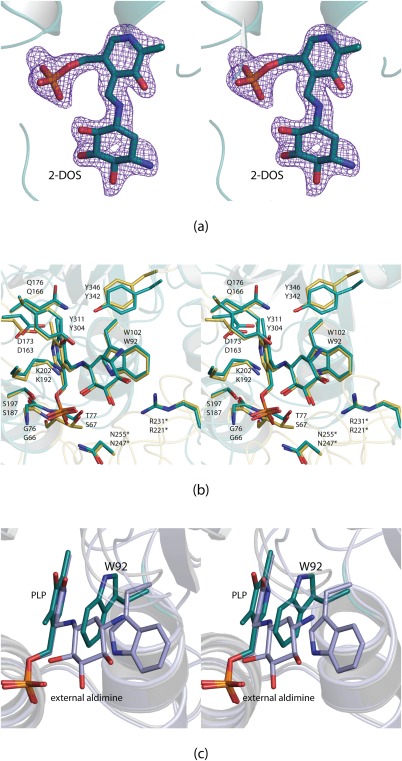
Structure of BtrR from B. circulans. Electron density corresponding to the bound external aldimine is shown in stereo in (a). It was calculated as described in the legend to Figure 1 and contoured at 4σ. A superposition of the BtrR and RbmB active sites is presented in (b). The RbmB and BtrR models are colored in yellow and teal, respectively. The top labels correspond to those amino acids in RbmB whereas the bottom labels refer to those found in BtrR. In the first structural analysis of BtrR, it was predicted that Trp 92 would have to swing away from the PLP cofactor.10 The hypothesis was, indeed, correct as shown in stereo in (c). The structure of BtrR with bound PLP is highlighted in teal whereas the structure of BtrR determined in this investigation is displayed in light blue.
