Fig. 2. Replacement of STIM1-TM by GpA-TM impairs the lumen-to-cytosol signal transmission.
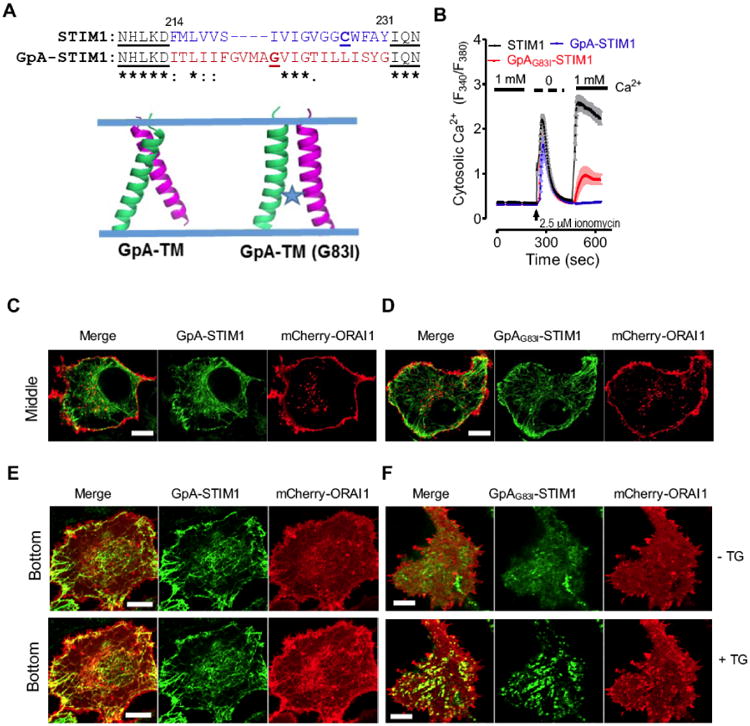
(A) A dimeric TM domain from glycophorin A (GpA; aa 73-94) was used to replace STIM1-TM (aa 214-230). Top, sequence alignment of STIM1-TM with GpA-TM; Bottom, modeled structures of a dimeric GpA-TM dimer. G83I is a well-known disruptive mutant of GpA that destabilizes its dimerization.
(B) Ca2+ response curves in HEK293-ORAI1-CFP stable cells transiently transfected with wild-type STIM1, chimeric GpA-STIM1 or the mutant GpAG83I-STIM1 monitored by ratiometric Fura-2 fluorescence (F340 nm/F380 nm). Store depletion was induced by 2.5 μM ionomycin. Shown were representative traces from three independent experiments (n = 3, 20∼30 cells per measurement). The solid bar above the curves indicates the presence of 1 mM Ca2+ in the external medium.
(C-D) Confocal images of the middle planes of HeLa cells co-expressing mCherry-ORAI1 and GFP-GpA-STIM1 (C) or the mutant GFP-GpAG83I-STIM1 (D). Both GpA-STIM1 and its mutant GpAG83I-STIM1 showed good ER distribution at rest. Scale bar, 5 μm.
(E-F) Confocal images of footprints of HeLa cells co-expressing mCherry-ORAI1 and GFP-GpA-STIM1 (E) or the mutant GFP-GpAG83I-STIM1 (F). 1 μM thapsigargin (TG) was added to trigger store depletion. Scale bar, 5 μm.
